(zye-DOH-vyou-deen)
Azidothymidine, AZT, Retrovir
Antiviral
pH 5.5
Usual dose
Treatment of HIV infection (symptomatic or asymptomatic):
1 mg/kg every 4 hours. Initiate oral therapy as soon as possible (100 mg PO approximately equal to 1 mg/kg IV). Impaired renal or hepatic function may increase toxicity. Usually part of a multidrug regimen; see Drug/Lab Interactions.
Prevention of maternal-fetal HIV transmission:
2 mg/kg of total body weight over 1 hour when labor begins. Follow with an infusion of 1 mg/kg/hr (of total body weight) until umbilical cord is clamped.
Pediatric dose
See Maternal/Child.
To avoid medication errors when preparing pediatric doses, use extra care to calculate the appropriate dose based on body weight (kg). Follow with oral therapy; see comments in Usual Dose.
Treatment of HIV infections in pediatric patients 4 weeks to less than 18 years of age, weight greater than 4 kg:
Recommendations for pediatric oral dosing (capsules, tablets, or syrup) can be found in the prescribing information. Should not exceed the recommended adult dose. See Dose Adjustments.
Neonatal dose
See Maternal/Child.
Treatment of HIV infections (unlabeled):
Oral dosing preferred but may be given IV.
Premature neonates (gestational age less than 30 weeks) younger than 6 weeks of age:
1.5 mg/kg IV every 12 hours. Increase to 2.3 mg/kg every 12 hours at 4 weeks of age.
Premature neonates (gestational age 30 to 35 weeks) younger than 6 weeks of age:
1.5 mg/kg IV every 12 hours. Increase to 2.3 mg/kg IV every 12 hours at 15 days of age.
Full-term infants (gestational age 35 weeks or more) younger than 6 weeks of age:
3 mg/kg IV every 12 hours.
Prevention of maternal-fetal HIV transmission:
Start neonatal dosing within 12 hours after birth and continue through 6 weeks of age. Oral dosing preferred but may be given IV. Consider impaired hepatic or renal function; see Precautions.
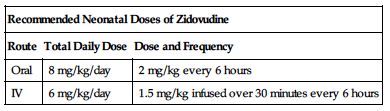
| Recommended Neonatal Doses of Zidovudine | ||
| Route | Total Daily Dose | Dose and Frequency |
| Oral | 8 mg/kg/day | 2 mg/kg every 6 hours |
| IV | 6 mg/kg/day | 1.5 mg/kg infused over 30 minutes every 6 hours |
Dose adjustments
Dose selection should be cautious in the elderly based on the potential for decreased organ function and concomitant disease or drug therapy. ■ Reduce dose to 1 mg/kg every 6 to 8 hours in patients with a CrCl less than 15 mL/min or in patients with ESRD who are being maintained on hemodialysis or peritoneal dialysis. ■ Dose reduction may be required in patients with hepatic impairment. Monitor for hematologic toxicity. ■ Dose interruption may be required in the presence of significant anemia or neutropenia. ■ Dose adjustment may be required in anemia and/or with other drugs. See Monitor, Drug/Lab Interactions, and Antidote.
Dilution
Each vial of zidovudine contains 200 mg in 20 mL (10 mg/mL). Withdraw the calculated dose and add to a sufficient volume of D5W to achieve a final concentration no greater than 4 mg/mL. To avoid medication errors when preparing a pediatric dose, use extra care to calculate the appropriate dose in mL based on body weight (kg).
Filters:
Not required by manufacturer; no further data available from manufacturer.
Storage:
Store undiluted vials at 15° to 25° C (59° to 77° F). Protect from light. Chemically and physically stable after dilution for 24 hours at RT or 48 hours refrigerated at 2° to 8° C (36° to 46° F). However, as an added precaution against microbial contamination, manufacturer recommends use within 8 hours if stored at 25° C (77° F) and 24 hours if refrigerated.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Manufacturer states, “Admixture in biologic or colloidal fluids (e.g., blood products, protein solutions) is not recommended.”
One source suggests the following compatibilities:
Additive:
Dobutamine, meropenem (Merrem IV), ranitidine (Zantac).
Y-site:
Acyclovir (Zovirax), allopurinol (Aloprim), amifostine (Ethyol), amikacin, amphotericin B (conventional), amphotericin B cholesteryl (Amphotec), anidulafungin (Eraxis), aztreonam (Azactam), ceftazidime (Fortaz), ceftriaxone (Rocephin), cisatracurium (Nimbex), clindamycin (Cleocin), dexamethasone (Decadron), dobutamine, docetaxel (Taxotere), dopamine, doripenem (Doribax), doxorubicin liposomal (Doxil), erythromycin (Erythrocin), etoposide phosphate (Etopophos), filgrastim (Neupogen), fluconazole (Diflucan), fludarabine (Fludara), gemcitabine (Gemzar), gentamicin, granisetron (Kytril), heparin, imipenem-cilastatin (Primaxin), linezolid (Zyvox), lorazepam (Ativan), melphalan (Alkeran), meropenem (Merrem IV), metoclopramide (Reglan), morphine, nafcillin (Nallpen), ondansetron (Zofran), oxacillin (Bactocill), oxytocin (Pitocin), paclitaxel (Taxol), pemetrexed (Alimta), pentamidine, phenylephrine (Neo-Synephrine), piperacillin/tazobactam (Zosyn), potassium chloride (KCl), ranitidine (Zantac), remifentanil (Ultiva), sargramostim (Leukine), sulfamethoxazole/trimethoprim, teniposide (Vumon), thiotepa, tobramycin, vancomycin, vinorelbine (Navelbine).
Rate of administration
Intermittent infusion:
Each single dose properly diluted must be delivered at a constant rate over 1 hour. Avoid rapid infusion or IV bolus.
Continuous infusion: Prevention of maternal-fetal HIV transmission:
2 mg/kg equally distributed over 1 hour, followed by 1 mg/kg/hr until umbilical cord clamped.
Neonates:
A single dose infused over 30 minutes.
Actions
A pyrimidine nucleoside analog active against HIV. Through a specific process this thymidine analog interferes with reverse transcriptase, thus inhibiting viral replication. Metabolized by glucuronidation in the liver and excreted through the kidneys. Oral dosing half-life is approximately 0.5 to 3 hours in adults. Crosses the placental barrier. Secreted in breast milk.
Indications and uses
Treatment of HIV-1 infection in combination with other antiretroviral agents. ■ Prevention of maternal-fetal HIV-1 transmission. In most cases, should be given in combination with other antiretroviral drugs. Protocol includes oral zidovudine beginning between week 14 and week 34 of gestation, continuing until labor begins, IV dosing during labor, and zidovudine syrup or IV dosing to the newborn; see Maternal/Child.
Contraindications
Life-threatening hypersensitivity reactions to any of the components.
Precautions
For IV use only. Do not give IM or SC. ■ Incidence of adverse reactions appears to increase with disease progression. ■ Has been associated with hematologic toxicity, including neutropenia and severe anemia, particularly in patients with advanced HIV. ■ Use with caution in patients with bone marrow compromise as indicated by a granulocyte count of less than 1,000/mm3 or hemoglobin below 9.5/dL. Hematologic toxicities appear to be related to pretreatment bone marrow reserve and to dose and duration of therapy. ■ Prolonged use of zidovudine has been associated with symptomatic myopathy and myositis with pathologic changes similar to those produced by HIV disease. ■ Lactic acidosis and severe hepatomegaly with steatosis have been reported with the use of some nucleoside analogs, including zidovudine when used alone or in combination. Deaths have occurred. Female gender, obesity, and prolonged exposure to antiretroviral nucleoside analogs may increase risk. Has also been reported in patients with no known risk factors. Exercise particular caution in any patient with hepatomegaly, hepatitis, or other risk factors for liver disease. Suspend therapy in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis, even in the absence of marked transaminase elevations). ■ Use with caution in patients with severe hepatic impairment; may be at increased risk for hematologic toxicity. ■ Hepatic decompensation has occurred in HIV/HCV co-infected patients receiving combination antiretroviral therapy for HIV and interferon alfa with or without ribavirin (Rebetol) for HCV. ■ Use with caution in patients with severely impaired renal function (CrCl less than 15 mL/min); see Dose Adjustments. ■ Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including zidovudine. Patients may develop an inflammatory response to indolent opportunistic infections (e.g., Mycobacterium avium, CMV, PCP, or tuberculosis). May require further evaluation and treatment. ■ Autoimmune disorders (e.g., Graves’ disease, polymyositis, and Guillain-Barré syndrome) have been reported in the setting of immune reconstitution; may occur months after initiation of therapy. ■ Vial stoppers contain natural rubber latex; may cause hypersensitivity reactions in latex-sensitive individuals. ■ See Drug/Lab Interactions.
Monitor:
Observe closely; not a cure for HIV infections. Patients may acquire illnesses associated with AIDS or AIDS-related complex (ARC), including opportunistic infections. ■ Frequent blood cell counts are required. Hematologic toxicity, including neutropenia, severe anemia, and occasionally reversible pancytopenia are common. Anemia may occur as early as 2 to 4 weeks. Neutropenia usually occurs after 6 to 8 weeks of therapy; dosage adjustments and/or transfusions may be required. ■ Monitor liver function. ■ Obtain baseline CD4 lymphocyte count and monitor as indicated. ■ Closely monitor patients receiving zidovudine and interferon alfa, with or without ribavirin (Rebetol), for treatment-associated toxicities, especially hepatic decompensation, neutropenia, and anemia. Discontinuation of zidovudine and dose reduction or discontinuation of interferon, ribavirin, or both may be required if worsening clinical toxicities are seen (e.g., hepatic decompensation [e.g., Child-Pugh score greater than 6]). ■ See Drug/Lab Interactions.
Patient education:
Zidovudine is not a cure. Remain under the care of a physician when using zidovudine, and avoid actions that can spread HIV-1 infection to others. ■ Report abdominal pain, jaundice, muscle weakness, shortness of breath, or rapid breathing promptly. ■ Major side effects are neutropenia and/or anemia. ■ Requires frequent lab work and close follow-up with physician; keep all appointments. ■ Check with physician before taking any other medications.
Maternal/child:
Category C: safety for use during pregnancy has been evaluated. Risk versus benefit appears justified only in HIV-infected mothers. Congenital deformities not increased in studies; however, long-term consequences of in utero and neonatal exposure to zidovudine are unknown. Because the fetus is most susceptible to the potential teratogenic effects of drugs during the first 10 weeks of gestation and because the risks of therapy with zidovudine during this period are not fully known, women in the first trimester of pregnancy who do not require immediate initiation of antiretroviral therapy for their own health may consider delaying use. (Indication for prevention of maternal-fetal HIV-1 transmission is based on use after 14 weeks of gestation.) ■ Prevention of HIV-1 transmission in women who have received zidovudine for a prolonged period before pregnancy has not been evaluated. ■ Discontinue breast-feeding to reduce incidence of HIV transmission and potential for serious adverse reactions in nursing infants. ■ Has been studied in HIV-infected pediatric patients over 6 weeks of age who have HIV-related symptoms or are asymptomatic with abnormal lab values, indicating significant HIV-related immunosuppression. Has also been studied in neonates perinatally exposed to HIV. ■ To monitor maternal-fetal outcomes, an Antiretroviral Pregnancy Registry has been established. See prescribing information.
Elderly:
Dose selection should be cautious; see Dose Adjustments. Consider impaired hepatic and renal function.
Drug/lab interactions
Do not use in combination with other products that contain zidovudine (e.g., lamivudine/zidovudine [Combivir], abacavir/lamivudine/zidovudine [Trizivir]). ■ Probenecid may inhibit glucuronidation or reduce renal excretion of zidovudine, increasing zidovudine toxicity. ■ Use with protease inhibitors (e.g., nelfinavir [Viracept], ritonavir [Norvir]) may decrease serum concentrations of zidovudine; monitoring indicated; dose adjustment of zidovudine is not indicated. ■ Use with atovaquone (Mepron), fluconazole (Diflucan), methadone (Dolophine), or valproic acid (Depacon, Depakene) may increase zidovudine serum concentrations; monitoring indicated; dose adjustment of zidovudine is not indicated. ■ Rifamycins (e.g., rifampin [Rifadin], rifabutin [Mycobutin]) may increase clearance, reduce zidovudine serum levels, and reduce effectiveness. ■ Antagonized by doxorubicin (Adriamycin), stavudine (Zerit), and ribavirin (Rebetol). Avoid concurrent use. ■ Exacerbation of anemia due to ribavirin has been seen in patients co-infected with HIV/HCV when zidovudine is part of the HIV regimen; coadministration is not advised. ■ Hematologic toxicity increased with ganciclovir (Cytovene), interferons, or other bone marrow suppressants or cytotoxic agents (e.g., amphotericin B [conventional], dapsone, doxorubicin [Adriamycin], flucytosine, interferon, pentamidine, ribavirin [Rebetol], vinblastine, vincristine). Close clinical and laboratory monitoring indicated if coadministration is necessary. ■ Phenytoin levels may increase or decrease; monitor carefully to ensure proper dosing. ■ Phenytoin may also increase zidovudine levels by decreasing clearance. ■ Use with acyclovir may cause neurotoxicity (drowsiness, lethargy). ■ Combination therapy with zidovudine and interferon alfa, with or without ribavirin (Rebetol), may increase risk of hepatic decompensation, neutropenia, and anemia. ■ Prescribing information states that dose modification is also not warranted with coadministration of clarithromycin (Biaxin), lamivudine (Epivir), probenecid, or rifampin (Rifadin). Other sources suggest drug adjustments with some adverse reactions.
Side effects
Frequency and severity of adverse events are greater in patients with more advanced infection at the time of initiation of treatment. Anorexia, headache, malaise, nausea, and vomiting are most common with oral and IV administration. Anemia and neutropenia were reported frequently with IV administration. Abdominal cramps, abdominal pain, anaphylaxis, anorexia, arthralgia, asthenia, chills, constipation, dyspepsia, fatigue, granulocytopenia, hepatomegaly (severe), hyperbilirubinemia, increased liver function tests (e.g., ALT, AST, alkaline phosphatase), injection site reaction (e.g., pain, redness), insomnia, musculoskeletal pain, myalgia, neuropathy, pancytopenia (reversible), and thrombocytopenia have also been reported. Side effects most commonly reported in pediatric patients were anemia, cough, fever, and neutropenia.
Antidote
Notify physician of all side effects; most will be treated symptomatically. Moderate anemia or granulocytopenia may respond to a reduction in dose. Interrupt zidovudine therapy for severe anemia (less than 7.5 Gm/dL or a 25% reduction from baseline) or severe granulocytopenia (less than 750/mm3 or 50% reduction from baseline). Transfusions may be required. If marrow recovery occurs following dose interruption, resumption of therapy may be appropriate using adjunctive measures such as epoetin alfa (Epogen). Suspend treatment if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity occur. Rash may be the first sign of a more serious reaction (e.g., anaphylaxis, Stevens-Johnson syndrome); notify physician and treat with diphenhydramine (Benadryl), epinephrine (Adrenalin), and corticosteroids as indicated. Antimicrobial therapy may be indicated to treat opportunistic infections. Not removed by hemodialysis or peritoneal dialysis.
Ziv-aflibercept 
(ZIV-a-FLIB-er-sept)
Zaltrap
Angiogenesis inhibitor
Vascular endothelial growth factor (VEGF) inhibitor
Antineoplastic
pH 6.2
Usual dose
4 mg/kg as an infusion over 1 hour every 2 weeks. Administer before any component of the FOLFIRI regimen (5-fluorouracil, leucovorin, and irinotecan) on the day of treatment. Continue until disease progression or unacceptable toxicity. See Monographs for 5-fluorouracil, leucovorin, and irinotecan to incorporate all requirements into this regimen.
Dose adjustments
| Ziv-Aflibercept Dose Adjustments* | |
| Indication | Recommended Dose or Action |
| Neutrophil count less than 1,500/mm3 (1.5 × 109/L) | Withhold or delay dosing until neutrophil count is at or above 1.5 × 109/L (1,500/mm3). |
| Elective surgery | Withhold for at least 4 weeks before elective surgery. |
| Recurrent or severe hypertension | Withhold until hypertension is controlled, then resume at a permanently reduced dose of 2 mg/kg. |
| Proteinuria at or above 2 Gm/24 hr | Withhold ziv-aflibercept. Resume when proteinuria decreases to less than 2 Gm/24 hr. |
| Recurrent proteinuria | Withhold ziv-aflibercept until proteinuria is less than 2 Gm/24 hr, then resume at a permanently reduced dose of 2 mg/kg. |
| Severe hemorrhage, GI perforation, compromised wound healing, fistula formation, hypertensive crisis or hypertensive encephalopathy, arterial thromboembolic events, nephrotic syndrome or thrombotic microangiopathy, and/or reversible posterior leukoencephalopathy syndrome (RPLS) | Discontinue ziv-aflibercept. |
| Age, gender, or race; mild, moderate, or severe renal impairment or mild or moderate hepatic impairment; see Precautions. | No dose adjustment required. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


