(ka-BAZ-i-TAX-el)
Jevtana
Antineoplastic agent (taxane)
Usual dose
Premedication:
Must be premedicated at least 30 minutes before each dose to reduce the risk and/or severity of hypersensitivity reactions. Usual regimen includes IV administration of an antihistamine (diphenhydramine [Benadryl] 25 mg or equivalent), a corticosteroid (dexamethasone [Decadron] 8 mg or equivalent steroid), and an H2 antagonist (ranitidine [Zantac] 50 mg or equivalent H2 antagonist).
Antiemetic prophylaxis is recommended and may be given orally or IV as needed.
Cabazitaxel:
25 mg/M2 as a 1-hour infusion every 3 weeks. Given in combination with oral prednisone 10 mg administered daily throughout cabazitaxel treatment.
Dose adjustments
Recommendations for dose adjustment for adverse reactions are listed in the following chart.
| Recommended Cabazitaxel Dose Modifications for Adverse Reactions | |
| Toxicity* | Dose Modification |
| Prolonged Grade ≥3 neutropenia (greater than 1 week) despite appropriate medication, including G-CSF | Delay treatment until neutrophil count is >1,500 cells/mm3, then reduce dose of cabazitaxel to 20 mg/M2. Use G-CSF for secondary prophylaxis. |
| Febrile neutropenia or neutropenic infection | Delay treatment until improvement or resolution and until neutrophil count is >1,500 cells/mm3, then reduce dose of cabazitaxel to 20 mg/M2. Use G-CSF for secondary prophylaxis. |
| Grade ≥3 diarrhea or persisting diarrhea despite appropriate medication and fluid and electrolytes replacement. | Delay treatment until improvement or resolution, then reduce dose of cabazitaxel to 20 mg/M2. |
| Grade 2 peripheral neuropathy | Delay treatment until improvement or resolution, then reduce dose of cabazitaxel to 20 mg/M2. |
| Grade ≥3 peripheral neuropathy | Discontinue cabazitaxel. |
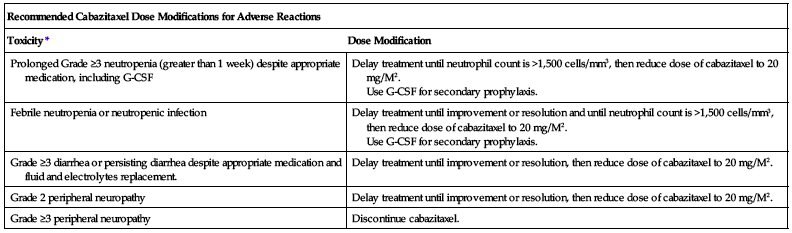
*Toxicities graded in accordance with National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Discontinue if any of these reactions continue at a cabazitaxel dose of 20 mg/M2. ■ Dosage modifications based on the degree of hepatic impairment are outlined in the following chart.
| Dose Modification for Hepatic Impairment | |
| Degree of Impairment | Dosage Modification |
| Mild hepatic impairment (total bilirubin >1 to ≤1.5 ULN or AST >1.5 × ULN) | Reduce starting dose of cabazitaxel to 20 mg/M2. |
| Moderate hepatic impairment (total bilirubin >1.5 to ≤3 ULN or AST = Any value) | Reduce starting dose of cabazitaxel to 15 mg/M2 based on tolerability data in these patients; however, the efficacy of this dose is unknown. |
| Severe hepatic impairment (total bilirubin >3 × ULN | Cabazitaxel is contraindicated; do not administer. |

■ Avoid coadministration with strong CYP3A inhibitors. If coadministration is required, consider a 25% cabazitaxel dose reduction; see Drug/Lab Interactions.
Dilution
Specific techniques required; see precautions.
Improper preparation has resulted in overdose. Must be diluted by a specific 2-step process and given as an infusion. Do not use PVC infusion containers and polyurethane infusion sets for preparation or administration. A clear yellow to brownish-yellow viscous solution.
Step 1:
Initially withdraw the entire contents of the provided diluent and inject into the vial of cabazitaxel (60 mg/1.5 mL). When transferring the diluent, direct the needle onto the inside wall of the cabazitaxel vial to limit foaming. Gently mix by repeated inversions for at least 45 seconds to ensure full mixing of drug and diluent. Do not shake. Allow to stand for a few minutes to allow most of the foam to dissipate. Should not contain visible particulate matter. Concentration of the resultant dilution is 10 mg/mL. This initially diluted solution should be used immediately but must be used within 30 minutes of entry into the vial.
Step 2:
Withdraw the recommended dose from the 10-mg/mL vial of cabazitaxel. Further dilute into a sterile 250-mL PVC-free container of either NS or D5W for infusion. If a dose greater than 65 mg is required, use a larger volume of NS or D5W infusion so a concentration of 0.26 mg/mL is not exceeded. Concentration of the final solution should be between 0.10 and 0.26 mg/mL. Thoroughly mix by gently inverting the bag or bottle.
Filters:
Use of a 0.22-micron in-line filter is required for administration (not supplied).
Storage:
Before use, store at 25° C (77° F), with excursions permitted between 15° to 30° C (59° to 86° F). Do not refrigerate. Initial diluted solution should be used immediately but must be used within 30 minutes; discard any unused portion. Final infusion solution should be used within 8 hours (including infusion time) at RT or within 24 hours (including infusion time) if refrigerated. Both solutions are supersaturated and may crystallize over time. Discard if crystallization occurs. Discard if either the initial diluted solution or final solution is not clear or appears to have precipitation.
Compatibility
Manufacturer states, “Do not use PVC infusion containers and polyurethane infusion sets for preparation or administration. Should not be mixed with any other drugs.”
Rate of administration
A single dose properly diluted as an infusion over 1 hour. Use of a 0.22-micron in-line filter required for administration. See Dilution and Compatibility.
Actions
An antineoplastic belonging to the taxane class. Prepared by semi-synthesis with a precursor extracted from yew needles. A microtubule inhibitor, it binds to tubulin and promotes its assembly into microtubules while simultaneously inhibiting disassembly. This leads to the stabilization of microtubules, which results in the inhibition of mitotic and interphase cellular functions. Highly protein bound (89% to 92%). Equally distributed between blood and plasma. Extensively metabolized in the liver, mainly by the CYP3A4/5 isoenzyme and to a lesser extent by CYP2C8. Terminal half-life is approximately 95 hours. Mainly excreted in feces as numerous metabolites with minimal excretion of unchanged drug in urine.
Indications and uses
Treatment of patients with hormone-refractory metastatic prostate cancer previously treated with a docetaxel-containing treatment regimen. Used in combination with oral prednisone.
Contraindications
Patients with neutrophil counts of equal to or less than 1,500/mm3. ■ Patients with a history of severe hypersensitivity reactions to cabazitaxel or other drugs formulated with polysorbate 80. ■ Patients with severe hepatic impairment (total bilirubin greater than 3 times the ULN); see Dose Adjustments and Precautions.
Precautions
Follow guidelines for handling cytotoxic agents. See Appendix A, p. 1331. ■ Usually administered by or under the direction of the physician specialist in a facility with adequate diagnostic and treatment facilities to monitor the patient and respond to any medical emergency. ■ If initial or fully diluted solution should come into contact with the skin or mucus, immediately and thoroughly wash with soap and water. ■ Bone marrow suppression manifested as neutropenia, anemia, thrombocytopenia, and/or pancytopenia may occur. Neutropenic deaths have been reported. Primary prophylaxis with G-CSF should be considered in high-risk patients (e.g., extensive prior radiation ports, over 65 years of age, poor nutritional status, poor performance status, previous episodes of febrile neutropenia, or other serious comorbidities that may result in increased complications from prolonged neutropenia). Therapeutic use of G-CSF and secondary prophylaxis should be considered in all patients considered to be at increased risk for neutropenia complications. ■ Use caution in patients with a hemoglobin less than 10 Gm/dL. ■ Severe hypersensitivity reactions have occurred; all patients are premedicated to reduce the risk and/or severity of a hypersensitivity reaction. Patients with a history of severe hypersensitivity reactions should not be rechallenged with cabazitaxel. ■ Nausea, vomiting, and severe diarrhea may occur. Deaths related to diarrhea and electrolyte imbalance have occurred. GI hemorrhage and perforation, ileus, enterocolitis, and neutropenic enterocolitis, including fatal outcome, have also been reported. Risk may be increased with neutropenia; age; steroid use; concomitant use of NSAIDs, antiplatelet therapy, or anticoagulants; and patients with a prior history of pelvic radiotherapy, adhesions, ulceration, and GI bleeding; see Drug/Lab Interactions. ■ Deaths due to renal failure have occurred, and most occurred in association with sepsis, dehydration, or obstructive uropathy. ■ Use with caution in patients with ESRD. ■ Use with caution in patients with mild to moderate hepatic impairment; see Dose Adjustments and Contraindications.
Monitor:
Obtain baseline CBC with differential and platelets. Monitor weekly during Cycle 1 and before each treatment cycle thereafter. ■ If febrile neutropenia or prolonged neutropenia (greater than 1 week) occurs despite appropriate medications (e.g., G-CSF), dose reduction is indicated. May not be restarted until the neutrophil count recovers to a level greater than 1,500 cells/mm3; see Contraindications and Dose Adjustments. ■ G-CSF may be administered to reduce the risks of neutropenia complications; see Precautions. ■ Monitor closely for hypersensitivity reactions, especially during the first and second infusions. May occur within the first few minutes, and beginning symptoms may include bronchospasm, generalized rash/erythema, and hypotension. ■ Obtain baseline and periodic bilirubin and liver function tests. Patients with mild to moderate hepatic impairment should be monitored closely. ■ Monitor for nausea, vomiting, and diarrhea. Use prophylactic antiemetics to reduce nausea and vomiting and increase patient comfort. Rehydrate and use antidiarrheal medications as indicated. ■ Monitor for S/S that may indicate serious GI toxicity (e.g., abdominal pain and tenderness, fever, persistent constipation, diarrhea, with or without neutropenia). Evaluate and treat promptly; see Antidote. ■ Monitor for S/S of impending renal failure (dehydration, reduced urine output). Monitoring of CrCl or SCr may be indicated. ■ Observe closely for signs of infection. Prophylactic antibiotics may be indicated pending results of C/S in a febrile neutropenic patient. ■ Monitor for thrombocytopenia (platelet count less than 50,000/mm3). Initiate precautions to prevent excessive bleeding (e.g., inspect IV sites, skin, and mucous membranes; use extreme care during invasive procedures; test urine, emesis, stool, and secretions for occult blood).
Patient education:
Avoid pregnancy; nonhormonal birth control recommended. ■ Routine monitoring of blood counts imperative. ■ Oral prednisone must be taken as prescribed. ■ Promptly report S/S of a hypersensitivity reaction (e.g., hives, rash, shortness of breath or troubled breathing, swelling of eyelids, lips, or face). ■ May cause severe and/or fatal infections, dehydration, and renal failure. Take your temperature often and promptly report a fever. ■ Promptly report burning on urination, cough, decreased urine output, hematuria, muscle aches, and/or significant diarrhea or vomiting. ■ Drug interactions may occur; review prescription and nonprescription drugs with your physician. ■ Certain side effects may be more frequent or severe in the elderly. ■ See Appendix D, p. 1333.
Maternal/child
(cabazitaxel is not indicated for female patients): Category D: avoid pregnancy. Can cause fetal harm. ■ Discontinue breast-feeding. ■ Safety and effectiveness for use in pediatric patients not established.
Elderly:
No overall differences in efficacy were observed. Patients over 65 years of age are more likely to experience fatal outcomes not related to disease progression and certain serious adverse reactions, including neutropenia and febrile neutropenia. The incidence of asthenia, dehydration, dizziness, fatigue, fever, and urinary tract infections was also increased in the elderly.
Drug/lab interactions
Formal drug interaction studies have not been conducted. ■ Prednisone administered at 10 mg daily did not affect the pharmacokinetics of cabazitaxel. ■ Primarily metabolized through CYP3A. Concomitant administration with strong CYP3A4 inhibitors (e.g., atazanavir [Reyataz], clarithromycin [Biaxin], indinavir [Crixivan], itraconazole [Sporanox], ketoconazole [Nizoral], nefazodone, nelfinavir [Viracept], ritonavir [Norvir], saquinavir [Invirase], telithromycin [Ketek], and voriconazole [VFEND]) should be avoided if possible. Plasma concentration of cabazitaxel is expected to increase. If concomitant administration is required, consider dose reduction; see Dose Adjustments. ■ Concomitant administration with rifampin (Rifadin), a strong CYP3A4 inducer, decreased the plasma concentration of cabazitaxel. Although no formal drug interactions have been conducted, it has been recommended that coadministration with strong CYP3A4 inducers (e.g., carbamazepine [Tegretol], phenytoin [Dilantin], phenobarbital [Luminal], rifabutin [Mycobutin], rifampin [Rifadin], and rifapentine [Priftin]) be avoided. ■ Avoid use of St. John’s wort; may also decrease concentration of cabazitaxel. ■ Cabazitaxel is not an inhibitor of CYP3A.
Side effects
Abdominal pain, alopecia, anorexia, arthralgia, asthenia, back pain, bone marrow suppression (e.g., anemia, leukopenia, neutropenia, thrombocytopenia), constipation, cough, diarrhea, dysgeusia, dyspnea, fatigue, fever, hematuria, nausea, peripheral neuropathy, and vomiting are most common. Serious side effects include gastrointestinal symptoms, hypersensitivity reactions, infection, neutropenia, febrile neutropenia, and renal failure. Cardiac arrhythmia, dehydration, dizziness, dyspepsia, dysuria, headache, hypotension, mucosal inflammation, muscle spasms, pain, peripheral edema, UTI, and weight loss have also occurred.
Post-marketing:
Gastritis and intestinal obstruction.
Overdose:
Exacerbation of adverse reactions such as bone marrow suppression and GI disorders.
Antidote
Keep physician informed of all side effects. Most will be treated symptomatically as indicated. Minor hypersensitivity reactions may subside with temporary discontinuation of cabazitaxel and additional antihistamines, corticosteroids, or H2 antagonists. Reduction in rate of administration may allow continued treatment. Discontinue immediately if severe hypersensitivity reactions occur, and administer appropriate therapy. Neutropenia can be profound but may be treated with filgrastim (Neupogen, Zarxio). Severe thrombocytopenia may require platelet transfusions. Severe anemia (less than 8 Gm/dL) may require packed cell transfusions; moderate anemia (less than 11 Gm/dL) may be treated with darbepoetin alfa (Aranesp) or epoetin alfa (Epogen). Severe GI toxicity may require cabazitaxel treatment delay or discontinuation. Discontinue cabazitaxel for Grade 3 or higher peripheral neuropathy or if adverse reactions continue after the dose adjustment to 20 mg/M2. There is no specific antidote for overdose. Administer G-CSF as soon as possible and closely monitor chemistry, vital signs, and other functions. Resuscitate if indicated.
Caffeine citrate
(KAF-feen SIT-rayt)
Cafcit
CNS stimulant
Respiratory stimulant adjunct
pH 4.7
Pediatric dose
The dose expressed as caffeine base is one half the dose when expressed as caffeine citrate. The recommended loading dose and maintenance dose are listed in the following chart.
| Guidelines for Loading and Maintenance Doses of Caffeine Citrate | ||
| Dose of Cafcit (volume) | Dose Expressed as Caffeine Citrate | |
| Loading dose | 1 mL/kg | 20 mg/kg for 1 dose |
| Maintenance dose | 0.25 mL/kg | 5 mg/kg every 24 hours |
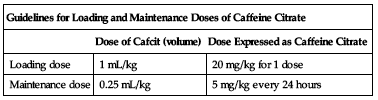
Begin maintenance dose 24 hours after loading dose. Maintenance dose may be given IV or orally. Duration of treatment beyond 10 to 12 days has not been studied. See Precautions.
Dose adjustments
May adjust maintenance dose using serum concentrations of caffeine. ■ To avoid toxicity in neonates with impaired renal or hepatic function, use with caution and monitor serum concentrations.
Dilution
Both the IV and the oral doses are supplied in vials containing 60 mg/3 mL of caffeine citrate (30 mg/3 mL of caffeine base). Confirm vial is for IV use. Withdraw calculated dose and dilute with sufficient D5W to administer at the recommended rate of administration.
Storage:
Store at CRT. Single-use vial. Discard any unused solution. Stable for 24 hours at room temperature when mixed with any of the solutions listed by the manufacturer in Compatibility.
Compatibility
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Solution:
Manufacturer lists D5W; another source adds D5/1/4NS, D5/1/4NS with 20 mEq KCl/L.
Additive:
Manufacturer lists amino acid solution 8.5% (Aminosyn 8.5%), calcium gluconate 10%, D5W, dextrose 50%, dopamine 40 mg/mL diluted to 0.6 mg/mL with D5W, fentanyl 50 mcg/mL diluted to 10 mcg/mL with D5W, heparin sodium 1,000 units/mL diluted to 1 unit/mL with D5W, IV fat emulsion 20% (Intralipid 20%).
One source suggests the following compatibilities:
Y-site:
Dopamine, doxapram (Dopram), fentanyl, heparin, levofloxacin (Levaquin).
Rate of administration
Use of a syringe pump infuser is recommended.
Loading dose:
Infuse over 30 minutes.
Maintenance dose:
Infuse over 10 minutes.
Actions
A bronchial smooth muscle relaxant, a CNS and cardiac stimulant, and a diuretic. Structurally related to other methylxanthines (e.g., theophylline and theobromine). Exact mechanism of action in apnea of prematurity is not known. Postulated mechanisms include stimulation of the respiratory center, increased minute ventilation, decreased threshold to hypercapnia, increased response to hypercapnia, increased skeletal muscle tone, decreased diaphragmatic fatigue, increased metabolic rate, increased oxygen consumption, blood vessel dilatation, central vessel vasoconstriction, and smooth muscle relaxation. Readily distributes into the brain. Caffeine levels in the CSF of preterm neonates approximate plasma levels. Metabolized in the liver by the cytochrome P450 system. Metabolism and elimination in the preterm neonate are much slower than in adults due to immature hepatic and/or renal function. Mean half-life and fraction excreted unchanged in the urine is inversely related to gestational/postconceptual age. In neonates, the half-life is approximately 3 to 4 days and the fraction excreted unchanged in the urine is approximately 86% (within 6 days). By 9 months of age, the metabolism of caffeine approximates that seen in adults (half-life is 5 hours and amount excreted unchanged is 1%). Interconversion between caffeine and theophylline has been reported in preterm neonates. After theophylline administration, caffeine levels are approximately 25% of theophylline levels. After caffeine administration, 3% to 5% of caffeine administered converts to theophylline.
Indications and uses
Short-term treatment of apnea of prematurity in infants more than 28 but less than 33 weeks’ gestational age. In one study apnea of prematurity was defined as having at least 6 apnea episodes of more than 20 seconds’ duration in a 24-hour period with no other identifiable cause of apnea.
Unlabeled uses:
Prevention of postoperative apnea in former preterm infants.
Contraindications
Hypersensitivity to any of its components.
Precautions
Reports in the literature suggest a possible association between the use of methylxanthines and the development of necrotizing enterocolitis. Necrotizing enterocolitis, resulting in death in some cases, has been reported in neonates receiving caffeine citrate. ■ Apnea of prematurity is a diagnosis of exclusion. Other causes of apnea (e.g., CNS disorders, primary lung disease, anemia, sepsis, metabolic disturbances, cardiovascular abnormalities, or obstructive apnea) should be ruled out or properly treated before initiating therapy with caffeine citrate. ■ Is a CNS stimulant. Use with caution in infants with seizure disorders. ■ May increase heart rate, left ventricular output, and stroke volume. Use with caution in infants with cardiovascular disease. ■ Duration of treatment of apnea of prematurity in trials has been limited to 10 to 12 days. Safety and efficacy of therapy beyond this time have not been established. ■ Safety and efficacy for use in prophylactic treatment of sudden infant death syndrome (SIDS) or before extubation in mechanically ventilated infants have not been established. ■ Use with caution in infants with impaired renal or hepatic function; see Dose Adjustments and Monitor. ■ Patients sensitive to other xanthines (e.g., aminophylline) may also be sensitive to caffeine.
Monitor:
Obtain baseline serum caffeine levels in infants previously treated with theophylline because preterm infants metabolize theophylline to caffeine; see Actions. Levels should also be obtained in infants born to mothers who ingested caffeine before delivery, as caffeine readily crosses the placenta. Caffeine levels ranged from 8 to 40 mg/L in clinical trials. A therapeutic plasma concentration range has not been determined, but one source suggests 5 to 25 mcg/mL. Serious toxicity has been reported at levels exceeding 50 mg/L. Monitor levels periodically during treatment to avoid toxicity. Monitoring is especially important in infants with impaired renal or hepatic function; see Dose Adjustments. ■ Monitor serum glucose periodically. Hypoglycemia and hyperglycemia have been reported. ■ Monitor for S/S of necrotizing enterocolitis (e.g., gastric distension, vomiting, bloody stools). Screening stools for occult blood may be helpful in identifying early-onset necrotizing enterocolitis.
Patient education:
Caregivers should be instructed to consult physician if infant continues to have apnea events and to not increase the dose of caffeine citrate without consulting a physician. ■ Contact physician at the first sign of lethargy or GI intolerance (e.g., abdominal distension, vomiting, or bloody stools). ■ Dose must be accurately measured, and any unused solution must be discarded.
Maternal/child:
Category C: no controlled studies; benefits should outweigh risks. ■ Half-life is increased in pregnant women. ■ Half-life may be in excess of 100 hours in infants under 6 months of age due to immature liver function.
Drug/lab interactions
Metabolized by the cytochrome P450 system. Lower caffeine doses may be required with coadministration of medications that inhibit the P450 system, decreasing the elimination of caffeine (e.g., cimetidine [Tagamet] and ketoconazole [Nizoral]). Higher caffeine doses may be needed with coadministration of medications that induce the P450 system, increasing the elimination of caffeine (e.g., phenobarbital and phenytoin). ■ Interconversion between caffeine and theophylline has been reported. Concurrent use of these drugs is not recommended; see Actions.
Side effects
Cardiovascular effects (e.g., tachycardia, increased left ventricular output, and increased stroke volume), CNS stimulation (e.g., irritability, jitteriness, restlessness), GI effects (e.g., feeding intolerance, gastritis, increased gastric aspirate, and necrotizing enterocolitis), hyperglycemia, hypoglycemia, renal effects (e.g., increased urine output, increased CrCl, and increased sodium and calcium excretion).
Overdose:
Signs and symptoms of caffeine overdose in the preterm infant may include: elevated BUN, elevated total leukocyte concentration, fever, fine tremor of extremities, hyperglycemia, hypertonia, insomnia, jitteriness, nonpurposeful jaw and lip movements, opisthotonos, seizures, tachypnea, tonic-clonic movements, or vomiting.
Antidote
Notify the physician of any side effects. Treatment of overdose is primarily symptomatic and supportive. Seizures may be treated with intravenous administration of diazepam (Valium) or a barbiturate such as pentobarbital (Nembutal). Caffeine levels have been shown to decrease after exchange transfusions. Resuscitate as necessary.
Calcitriol
(kal-si-TRYE-ole)
Calcijex
Vitamin D
pH 5.9 to 7
Usual dose
Effectiveness of calcitriol therapy is dependent on adequate daily intake of calcium. The RDA of calcium in adults is 800 mg. Calcium supplementation or proper dietary measures must be initiated and maintained.
Hypocalcemia and/or secondary hyperparathyroidism:
Recommended initial dose, depending on the severity of hypocalcemia and/or secondary hyperparathyroidism, is 1 mcg (0.02 mcg/kg) to 2 mcg administered at each hemodialysis treatment (three times weekly, approximately every other day). Initial doses have ranged from 0.5 to 4 mcg three times weekly.
Information supplied by the manufacturer suggests that the relative dosing of paricalcitol to calcitriol is 4:1. When converting a patient from calcitriol to paricalcitol, the initial dose of paricalcitol should be four times greater than the patient’s dose of calcitriol.
Pediatric dose
Hypocalcemia in end-stage renal disease (ESRD) (unlabeled):
0.01 to 0.05 mcg/kg/dose given 3 times a week; see Maternal/Child. See all comments under Usual Dose.
Dose adjustments
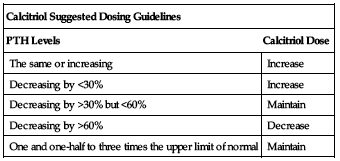
Adjust dosing based on patient response. Begin dosing at lower end of dose range in the elderly; see Elderly. If a satisfactory response is not observed, dose may be increased by 0.5 to 1 mcg at 2- to 4-week intervals. Monitor serum calcium, phosphorus, and calcium × phosphorus product (Ca × P) frequently during any dose adjustment period; see Monitor. ■ Discontinue therapy if elevated calcium level or a Ca × P product of greater than 70 is noted. Re-initiate therapy at a lower dose when parameters normalize. ■ Calcitriol dose may need to be reduced as the parathyroid hormone (PTH) levels decrease in response to therapy. The currently accepted target range for intact parathyroid hormone (iPTH) in chronic renal failure (CRF) patients is no more than 1.5 to 3 times the non-uremic upper limit of normal. Incremental dosing must be individualized and commensurate with PTH, serum calcium, and phosphorus levels. The following chart is a suggested approach to dose titration.
| Calcitriol Suggested Dosing Guidelines | |
| PTH Levels | Calcitriol Dose |
| The same or increasing | Increase |
| Decreasing by <30% | Increase |
| Decreasing by >30% but <60% | Maintain |
| Decreasing by >60% | Decrease |
| One and one-half to three times the upper limit of normal | Maintain |
Dilution
May be given undiluted. Available in 1-mcg/mL ampules.
Storage:
Store ampules at CRT. Protect from light. Calcitriol may be drawn up into a syringe up to 8 hours before administration but must be protected from direct sunlight.
Compatibility
Y-site:
D5W, NS, SWFI.
Rate of administration
Administer as a bolus dose into the venous line at the end of hemodialysis.
Actions
The active form of vitamin D3 (cholecalciferol). Must be metabolically activated in liver and kidney before it is fully active on its target tissues. In bone, acts with PTH to stimulate resorption of calcium. In kidneys, increases tubular reabsorption of calcium. Stimulates intestinal calcium transport and directly suppresses synthesis and release of PTH from the parathyroid gland. A vitamin D–resistant state may exist in uremic patients because of the failure of the kidney to adequately convert precursors to the active compound, calcitriol. Duration of action is 3 to 5 days.
Indications and uses
Management of hypocalcemia in patients undergoing chronic renal dialysis. Has been shown to significantly reduce elevated PTH levels, which results in improvement in renal osteodystrophy.
Contraindications
Patients with hypercalcemia or evidence of vitamin D toxicity.
Precautions
Because calcitriol is the most potent form of vitamin D available, oral vitamin D supplements should be discontinued during treatment. ■ Dietary phosphorus should be restricted and a non–aluminum phosphate–binding compound (e.g., calcium acetate [PhosLo], sevelamer [Renagel]) should be administered to control serum phosphorus levels in patients undergoing dialysis.
Monitor:
Obtain baseline serum calcium, phosphorus, aluminum, albumin, and PTH assays. ■ Serum calcium levels should be corrected for serum albumin using the following equation: Corrected Ca = observed Ca + 0.8 × (normal albumin − observed albumin). For example: If serum calcium is 7 mg/dL and observed albumin is 2.5 Gm/dL, Corrected Ca = 7 + 0.8 × (4 − 2.5) = 8.2 mg/dL. All decisions regarding therapy should be based on corrected calcium values. ■ Monitor magnesium, alkaline phosphatase, and 24-hour urinary calcium and phosphorus periodically. ■ Criteria used to determine if calcitriol should be administered include: serum calcium less than 11.5 mg/dL, Ca × P less than 70, serum albumin less than 60 mcg/L (within normal limits), and PTH more than 3 times the upper limit of normal. ■ Serum calcium, phosphorus, and the Ca × P product should be monitored twice weekly during dose titration. Once stable, decrease monitoring to once monthly. See Dose Adjustments. ■ PTH levels, once stable, should be monitored every 3 months. Adynamic bone lesions may develop if PTH levels are suppressed to abnormal levels. If PTH levels fall below the target range (1.5 to 3 times the upper limit of normal), the calcitriol dose should be reduced. Discontinuation may result in rebound effect. Therefore gradual titration downward to a new maintenance dose is recommended; see Dose Adjustments. ■ Overdosage of vitamin D is dangerous. May induce hypercalcemia and/or hypercalciuria. If clinically significant hypercalcemia develops, dose should be reduced or held. Chronic hypercalcemia can lead to generalized vascular calcification, nephrocalcinosis, and other soft-tissue calcification. The serum Ca × P product should not be allowed to exceed 70. Radiographic evaluation of suspect anatomic regions may be useful in early detection of this condition; see Side Effects and Antidote. ■ Use with caution in patients receiving digoxin. Hypercalcemia may precipitate cardiac arrhythmias; see Drug/Lab Interactions.
Patient education:
Report symptoms of hypercalcemia promptly. Dose adjustment or treatment may be required. Strict adherence to dietary supplementation of calcium and restriction of phosphorus is required to ensure optimal effectiveness of therapy. Phosphate-binding compounds (e.g., calcium acetate [PhosLo]) may be needed to control serum phosphorus levels in patients with CRF, but excessive use of aluminum-containing products (e.g., aluminum hydroxide gel [Alternagel]) should be avoided. ■ Avoid use of unapproved nonprescription medications, including magnesium-containing antacids.
Maternal/child:
Category C: safety for use in pregnancy not established. Benefits must outweigh risks. ■ Safety for use in breast-feeding not established. A decision should be made whether to discontinue nursing or to discontinue the drug. ■ Safety and effectiveness have been studied in a small number of pediatric patients, ages 13 to 18 years, with ESRD on hemodialysis. The mean weekly dose ranged from 1 to 1.4 mcg. Use in this study program appeared to be safe and effective. See package insert for study information.
Elderly:
Begin dosing at lower end of dose range. Consider age-related organ impairment, concomitant disease, and/or drug therapy; see Dose Adjustments.
Drug/lab interactions
Specific interaction studies have not been performed. ■ Digoxin toxicity is potentiated by hypercalcemia. Use caution when calcitriol is prescribed concomitantly with digoxin compounds. ■ Phosphate or vitamin D–related compounds should not be taken concomitantly with calcitriol. ■ Magnesium-containing antacids and calcitriol should not be used concomitantly. Hypermagnesemia may result.
Side effects
Overdose or chronic administration may lead to hypercalcemia, hypercalciuria, and hyperphosphatemia. High intake of calcium and phosphate concomitant with calcitriol therapy may lead to similar abnormalities. Signs and symptoms of vitamin D intoxication associated with hypercalcemia include Early: bone pain, constipation, dry mouth, headache, metallic taste, muscle pain, nausea, somnolence, vomiting, and weakness. Late: albuminuria, anorexia, cardiac arrhythmias, conjunctivitis (calcific), decreased libido, ectopic calcification, elevated AST and ALT, elevated BUN, hypercholesterolemia, hypertension, hyperthermia, nocturia, overt psychosis (rare), pain at injection site, pancreatitis, photophobia, polydipsia, polyuria, pruritus, rhinorrhea, and weight loss. Rare cases of hypersensitivity reactions, including anaphylaxis, have been reported.
Antidote
Notify physician of any side effects. Treatment of patients with clinically significant hypercalcemia (more than 1 mg/dL above the upper limit of normal range) consists of supportive measures, immediate dose reduction or interruption of therapy, initiation of a low-calcium diet, withdrawal of calcium supplements, patient mobilization, attention to fluid and electrolyte imbalances, assessment of electrocardiographic abnormalities (critical in patients receiving digoxin), and hemodialysis or peritoneal dialysis against a calcium-free dialysate, as warranted. Hypercalcemia usually resolves in 2 to 7 days. Monitor serum calcium levels frequently until calcium levels return to within normal limits. May re-initiate calcitriol therapy at a dose 0.5 mcg less than prior dose. See Dose Adjustments.
Calcium chloride
(KAL-see-um KLOR-eyed)
Electrolyte replenisher
Antihypocalcemic
Cardiotonic
Antihyperkalemic
Antihypermagnesemic
pH 5.5 to 7.5
Usual dose
In a 10% solution, 10 mL (1 Gm) contains 13.6 mEq (272 mg) of calcium; 1 mL (100 mg), 1.36 mEq (27.2 mg). All doses based on a 10% solution.
Hypocalcemic disorders (prophylaxis, treatment, electrolyte replacement, maintenance):
5 to 10 mL (500 mg to 1 Gm) at intervals of 1 to 3 days. Repeat doses may be required and are based on patient response or serum calcium levels. May be given as part of a TPN program.
Magnesium intoxication:
5 mL (500 mg). Observe for signs of recovery before giving any additional calcium.
Hyperkalemia ECG disturbances of cardiac function:
1 to 10 mL (100 mg to 1 Gm); titrate dose by monitoring ECG changes. AHA guidelines recommend 5 to 10 mL of a 10% solution (500 mg to 1 Gm). Repeat as needed.
Cardiac resuscitation (see indications for specific uses):
0.02 to 0.04 mL/kg (2 to 4 mg/kg); repeat at 10-minute intervals as indicated or as measured by serum deficits of calcium. Consider need for calcium (usually gluconate or gluceptate) for every 500 mL of whole blood if arrest occurs in a situation requiring copious blood replacement.
Overdose of calcium channel blockers or beta adrenergic blockers (unlabeled):
AHA guidelines recommend 5 to 10 mL of a 10% solution (500 mg to 1 Gm). May repeat as needed.
Pediatric dose
Do not administer into a scalp vein in pediatric patients; see Precautions, Monitor, and Maternal/Child.
Hypocalcemic disorders:
0.027 to 0.05 mL/kg of body weight (2.7 to 5 mg/kg) of a 10% solution. Up to 10 mL (1 Gm)/day may be required. No data from clinical trials are available regarding repeat doses. Sources suggest repeat doses every 4 to 6 hours based on patient response or serum calcium levels.
Cardiac resuscitation (see indications for specific uses):
0.2 mL/kg of a 10% solution (20 mg/kg). Repeat as indicated at 10-minute intervals. AHA guidelines recommend consideration of this dose for documented or suspected hypocalcemia or hyperkalemia as well as for hypomagnesemia and calcium channel blocker overdose.
Dilution
May be given undiluted, but preferably diluted with an equal amount of SWFI or NS for injection to make a 5% solution. Solution should be warmed to body temperature. May be further diluted with most common infusion solutions and given as an intermittent or continuous infusion.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Calcium salts not generally mixed with carbonates, phosphates, sulfates, or tartrates. Extreme caution and a specific multistep process are required when calcium and phosphates are combined in parenteral nutrition solutions. Consult pharmacist.
One source suggests the following compatibilities:
Additive:
Amikacin, ascorbic acid, chloramphenicol (Chloromycetin), dobutamine, dopamine, hydrocortisone sodium succinate (Solu-Cortef), isoproterenol (Isuprel), lidocaine, magnesium sulfate, norepinephrine (Levophed), penicillin G potassium and sodium, pentobarbital (Nembutal), phenobarbital (Luminal), sodium bicarbonate, verapamil.
Y-site:
Amiodarone (Nexterone), ceftaroline (Teflaro), dobutamine, doxapram (Dopram), epinephrine (Adrenalin), esmolol (Brevibloc), 6% hydroxyethyl starch (Voluven), micafungin (Mycamine), milrinone (Primacor), morphine, nitroprusside sodium (Nitropress), paclitaxel (Taxol).
Rate of administration
0.5 to 1 mL of solution over 1 minute. Administration into a central or deep vein is preferred; see Monitor. Stop or slow infusion rate if patient complains of discomfort. Do not exceed the equivalent of 1 mL calcium chloride/minute by IV injection or infusion. Rapid administration may cause bradycardia; heat waves; local burning sensation; metallic, calcium, or chalky taste; moderate drop in BP; peripheral vasodilation; or a sense of oppression.
Actions
Calcium is a basic element prevalent in the human body. It affects bones, nerves, muscles, glands, cardiac and vascular tone, and normal coagulation of the blood. It is excreted in the urine and feces.
Indications and uses
Calcium preparations other than calcium chloride are often preferred except in cardiac resuscitation or calcium channel blocker toxicity. ■ Increase plasma calcium levels in hypocalcemic disorders (e.g., tetany [neonatal, parathyroid deficiency], vitamin D deficiency, alkalosis, conditions associated with intestinal malabsorption). ■ Treat ECG disturbances caused by hyperkalemia. ■ Adjunctive therapy in sensitivity reactions (especially with urticaria), insect bites or stings (relieve muscle cramping), acute symptoms of lead colic, rickets, or osteomalacia. ■ Cardiac resuscitation only to treat hypocalcemia, hyperkalemia, or calcium-channel blocker toxicity (verapamil, diltiazem), or after open heart surgery if epinephrine does not produce effective myocardial contractions. ■ Antidote for cardiac and respiratory depression of magnesium sulfate toxicity.
Unlabeled uses:
Treatment of arrhythmias and/or hypotension caused by an overdose of calcium channel blockers (e.g., diltiazem [Cardizem], verapamil) or beta-adrenergic blockers (e.g., atenolol [Tenormin], metoprolol [Lopressor], propranolol). ■ Prevention of hypotension caused by calcium channel blockers.
Contraindications
Digitalized patients, hypercalcemia, ventricular fibrillation. Not recommended in the treatment of asystole and electromechanical dissociation.
Precautions
Three times more potent than calcium gluconate. ■ For IV use only. ■ See Drug/Lab Interactions.
Monitor:
Confirm patency of vein; select a large vein and use a small needle to reduce vein irritation. Administration into a central or deep vein is preferred. Necrosis and sloughing will occur with IM or SC injection or extravasation. ■ Keep patient recumbent after injection to prevent postural hypotension. ■ Monitor vital signs carefully. ■ Monitor serum calcium levels as indicated. May cause hyperchloremic acidosis.
Maternal/child:
Category C: safety for use in pregnancy and breast-feeding not established. Use only when clearly needed. ■ Rarely used IV in pediatric patients. Use of a less irritating salt preferred because of small vein size.
Drug/lab interactions
Will increase digoxin toxicity and may cause arrhythmias. If necessary, give small amounts very slowly. ■ Potentiated by thiazide diuretics (e.g., chlorothiazide [Diuril]); may cause hypercalcemia or calcium toxicity. ■ May reduce plasma levels of atenolol (Tenormin). ■ Can reduce neuromuscular paralysis and respiratory depression produced by antibiotics such as kanamycin. ■ Antagonizes verapamil; can reverse clinical effects. ■ May cause metabolic alkalosis and inhibit binding of potassium with sodium polystyrene sulfonate.
Side effects
Usual doses will produce a local burning sensation, moderate drop in BP, and peripheral vasodilation. May cause bradycardia; cardiac arrest; heat waves; metallic, calcium, or chalky taste; prolonged state of cardiac contraction; sense of oppression; or tingling sensation, especially with a too-rapid rate of administration.
Overdose:
Coma, intractable nausea and vomiting, lethargy, markedly elevated plasma calcium level, weakness, and sudden death.
Antidote
If side effects occur, further dilution and decrease in the rate of administration may be necessary. If side effects persist, discontinue the drug and notify the physician. IV infusion of sodium chloride (to maintain normovolemia) and furosemide (Lasix) 80 to 100 mg IV every 2 to 4 hours (with caution) is recommended in overdose. Sodium chloride competes with calcium for reabsorption in the renal tubules; furosemide enhances the activity. Together they will reduce hypercalcemia by causing a marked increase in calcium excretion. Monitoring of fluid, electrolytes, and cardiac and respiratory status is imperative. Disodium edetate may be used with extreme caution as a calcium chelating agent if overdose is critical. For extravasation inject affected area with 1% procaine hydrochloride and hyaluronidase to reduce venospasm and dilute calcium. Use a 27- or 25-gauge needle. Warm, moist compresses may be helpful. Resuscitate as necessary.
Calcium gluconate
(KAL-see-um GLOO-koh-nayt)
Electrolyte replenisher
Antihypocalcemic
Cardiotonic
Antihyperkalemic
Antihypermagnesemic
pH 6 to 8.2
Usual dose
All doses based on a 10% solution, which yields 10 Gm/100 mL or 100 mg/mL.
Hypocalcemia disorders/maintenance:
2 to 15 Gm/24 hr in divided doses every 6 hours (0.5 to 3.75 Gm every 6 hours). Larger amounts may be given as an intermittent or continuous IV infusion. 10 mL (1 Gm in a 10% solution) contains 4.65 mEq (93 mg) of calcium.
Cardiac resuscitation (see indications for specific uses):
500 to 800 mg/dose. Repeat at 10-minute intervals as indicated by clinical condition or serum calcium level.
Hypocalcemic tetany:
1 to 3 Gm IV over 10 to 30 minutes; repeat in 6 hours if indicated.
Magnesium intoxication:
500 to 800 mg/dose (maximum dose 3 Gm). Repeat as indicated by patient response; observe for signs of recovery before giving additional calcium.
Hypocalcemia secondary to citrated blood infusion:
500 mg to 1 Gm/500 mL of citrated blood (infuse into another vein).
Pediatric dose
Do not administer into a scalp vein in pediatric patients; see Precautions/Monitor. All doses based on a 10% solution.
Hypocalcemia disorders/maintenance:
200 to 500 mg/kg/24 hr in divided doses every 6 hours (50 to 125 mg/kg [0.5 to 1.25 mL/kg] every 6 hours).
Hypocalcemic tetany:
100 to 200 mg/kg/dose (1 to 2 mL/kg) IV over 5 to 10 minutes. Repeat dose 6 hours later if indicated. Maximum dose 500 mg/kg/24 hr.
Cardiac resuscitation (see indications for specific uses):
100 mg/kg/dose (1 mL/kg/dose) IV every 10 minutes. AHA guidelines recommend 0.6 to 1 mL/kg of a 10% solution (60 to 100 mg/kg). Repeat if indicated. AHA guidelines recommend consideration of this dose for documented or suspected hypocalcemia or hyperkalemia as well as for hypomagnesemia and calcium channel blocker overdose.
Neonatal dose
Do not administer into a scalp vein in neonates; see Precautions/Monitor.
Hypocalcemia disorders/maintenance:
200 to 800 mg/kg/24 hr in divided doses every 6 hours (50 to 200 mg/kg/dose [0.5 to 2 mL/kg/dose] every 6 hours).
Hypocalcemic tetany:
100 to 200 mg/kg/dose (1 to 2 mL/kg) IV over 5 to 10 minutes. Repeat dose 6 hours later if indicated; maximum dose 500 mg/kg/24 hr.
Dilution
May be given undiluted or may be further diluted in up to 1,000 mL of NS for infusion. Solution should be warmed to body temperature. Solution must be clear and free of crystals. Crystals can be dissolved by heating to 80° C (146° F) in a dry heat oven for at least 1 hour. Shake vigorously; cool to room temperature. Discard if crystals persist.
Pediatric and neonatal dilution:
Must be further diluted with NS.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Calcium salts not generally mixed with carbonates, phosphates, sulfates, or tartrates. Extreme caution and a specific multistep process are required when calcium and phosphates are combined in parenteral nutrition solutions. Consult pharmacist.
One source suggests the following compatibilities:
Additive:
Amikacin, aminophylline, ascorbic acid, chloramphenicol (Chloromycetin), furosemide (Lasix), heparin, hydrocortisone sodium succinate (Solu-Cortef), lidocaine, magnesium sulfate, norepinephrine (Levophed), penicillin G potassium and sodium, phenobarbital (Luminal), potassium chloride (KCl), prochlorperazine (Compazine), tobramycin, vancomycin, verapamil.
Y-site:
Aldesleukin (Proleukin), allopurinol (Aloprim), amifostine (Ethyol), amiodarone (Nexterone), ampicillin, aztreonam (Azactam), bivalirudin (Angiomax), cefazolin (Ancef), ceftaroline (Teflaro), ciprofloxacin (Cipro IV), cisatracurium (Nimbex), cladribine (Leustatin), dexmedetomidine (Precedex), dobutamine, docetaxel (Taxotere), doripenem (Doribax), doxapram (Dopram), doxorubicin liposomal (Doxil), enalaprilat (Vasotec IV), epinephrine (Adrenalin), etoposide phosphate (Etopophos), famotidine (Pepcid IV), fenoldopam (Corlopam), filgrastim (Neupogen), gemcitabine (Gemzar), granisetron (Kytril), heparin, hetastarch in electrolytes (Hextend), hydrocortisone sodium succinate (Solu-Cortef), 6% hydroxyethyl starch (Voluven), labetalol, linezolid (Zyvox), melphalan (Alkeran), meropenem (Merrem IV), micafungin (Mycamine), midazolam (Versed), milrinone (Primacor), nicardipine (Cardene IV), oxaliplatin (Eloxatin), piperacillin/tazobactam (Zosyn), potassium chloride (KCl), prochlorperazine (Compazine), propofol (Diprivan), remifentanil (Ultiva), sargramostim (Leukine), tacrolimus (Prograf), telavancin (Vibativ), teniposide (Vumon), thiotepa, vinorelbine (Navelbine).
Rate of administration
In all situations stop or slow infusion rate if patient complains of discomfort. Rapid administration may cause vasodilation, decreased BP, cardiac arrhythmias, syncope, and cardiac arrest.
IV injection:
Undiluted, each 0.5 mL or fraction thereof over 1 minute. Do not exceed 1 mL/min (100 mg). 100 mg over 10 to 20 seconds in cardiac arrest.
Intermittent IV:
Do not exceed a rate of 100 mg/min (IV injection rate).
Infusion:
Do not exceed 120 to 240 mg/kg/hr with a maximum concentration of 50 mg/mL.
Pediatric and neonatal rate of administration:
Slow rate of administration considerably. Observe continuously.
Actions
Calcium is a basic element prevalent in the human body. It affects bones, nerves, glands, cardiac and vascular tone, and normal coagulation of the blood. It crosses the placental barrier and is secreted in breast milk. It is excreted in the urine and feces.
Indications and uses
Increase plasma calcium levels in hypocalcemic disorders (e.g., tetany [neonatal, parathyroid deficiency], vitamin D deficiency, alkalosis, conditions associated with intestinal malabsorption). ■ Adjunctive therapy in sensitivity reactions (especially with urticaria), insect bites or stings (relieves muscle cramping), acute symptoms of lead colic, rickets, or osteomalacia. ■ Cardiac resuscitation only to treat hypocalcemia, hyperkalemia, or calcium-channel blocker overdose (verapamil, diltiazem). ■ Antidote for cardiac and respiratory depression of magnesium sulfate toxicity. ■ Prevention of hypocalcemia during exchange transfusions. ■ Decrease capillary permeability in allergic conditions, nonthrombocytopenic purpura, and exudative dermatoses (e.g., dermatitis herpetiformis). ■ Treat pruritus of eruptions caused by drugs. ■ Treat ECG disturbances caused by hyperkalemia or verapamil-induced hypotension.
Unlabeled uses:
Treatment of verapamil overdose, acute hypotension from verapamil, and prevention of initial hypotension when it could be detrimental to a specific patient and verapamil is required.
Contraindications
IM use in infants and small children. Digitalized patients, hypercalcemia, ventricular fibrillation.
Precautions
Has only one third the potency of calcium chloride. ■ For IV use only; IM use permitted in adults only if IV administration cannot be accomplished; see Monitor.
Monitor:
Confirm patency of vein; select a large vein and use a small needle to reduce vein irritation. Local necrosis and abscess formation can occur with IM or SC injection or extravasation. ■ Keep patient recumbent after injection to prevent postural hypotension. ■ Monitor vital signs carefully.
Maternal/child:
Category C: safety for use in pregnancy not established; benefits must outweigh risk. ■ See Contraindications.
Drug/lab interactions
Will increase digoxin toxicity and may cause arrhythmias. If necessary, give small amounts very slowly. ■ Potentiated by thiazide diuretics (e.g., chlorothiazide [Diuril]); may cause hypercalcemia or calcium toxicity. ■ May reduce plasma levels of atenolol (Tenormin). ■ Antagonizes verapamil; can reverse clinical effects. ■ May cause metabolic alkalosis and inhibit binding of potassium with sodium polystyrene sulfonate.
Side effects
Rare when given as recommended: bradycardia; cardiac arrest; cardiac arrhythmias; heat waves; hypotension; metallic, calcium, or chalky taste; sense of oppression; syncope; tingling; and vasodilation can occur with too-rapid rate of administration. Depression of neuromuscular function, flushing, prolonged state of cardiac contraction can occur.
Overdose:
Coma, intractable nausea and vomiting, lethargy, markedly elevated plasma calcium level, weakness, and sudden death.
Antidote
If side effects occur, further dilution and decrease in the rate of administration may be necessary. If side effects persist, discontinue the drug and notify the physician. IV infusion of sodium chloride (to maintain normovolemia) and furosemide (Lasix) 80 to 100 mg IV every 2 to 4 hours (with caution) is recommended in overdose. Sodium chloride competes with calcium for reabsorption in the renal tubules; furosemide enhances the activity. Together they will reduce hypercalcemia by causing a marked increase in calcium excretion. Monitoring of fluid, electrolytes, and cardiac and respiratory status is imperative. Disodium edetate may be used with extreme caution as a calcium chelating agent if overdosage is critical. For extravasation inject affected area with 1% procaine hydrochloride and hyaluronidase to reduce venospasm and dilute calcium. Use a 27- or 25-gauge needle. Warm, moist compresses may be helpful. Resuscitate as necessary.
Cangrelor
(KAN-grel-or)
Kengreal
Antiplatelet agent
Usual dose
Cangrelor:
30 mcg/kg as an IV bolus followed immediately by a 4 mcg/kg/min IV infusion. Initiate the bolus infusion prior to percutaneous coronary intervention (PCI). The maintenance infusion should ordinarily be continued for at least 2 hours or for the duration of PCI, whichever is longer.
Transitioning to oral p2y12 therapy:
To maintain platelet inhibition after discontinuation of cangrelor infusion, an oral P2Y12 platelet inhibitor should be administered. Administer one as described below:
Ticagrelor (brilinta):
180 mg at any time during cangrelor infusion or immediately after discontinuation.
Prasugrel (effient):
60 mg immediately after discontinuation of cangrelor. Do not administer prasugrel before discontinuation of cangrelor.
Clopidogrel (plavix):
600 mg immediately after discontinuation of cangrelor. Do not administer clopidogrel before discontinuation of cangrelor.
Dose adjustments
No dose adjustments needed based on sex, age, renal status, or hepatic function. ■ The impact of weight on drug exposure is accounted for by the use of weight-based dosing.
Dilution
Available as a sterile lyophilized powder in single-use 10-mL vials containing 50 mg cangrelor. Reconstitute each 50-mg vial with 5 mL of SWFI. Swirl gently until all material is dissolved. Avoid vigorous mixing. Allow any foam to settle. Ensure that the contents of the vial are fully dissolved and the reconstituted material is a clear, colorless to pale yellow solution. Before administration, each reconstituted vial must be further diluted with NS or D5W. Withdraw the contents from the reconstituted vial and add to a 250-mL bag of NS or D5W. Mix the bag thoroughly. Final concentration is 200 mcg/mL and should be sufficient for at least 2 hours of dosing. Patients 100 kg and over will require a minimum of 2 bags.
Storage:
Store vials of cangrelor at CRT (20° to 25° C [68° to 77° F]), with excursions between 15° and 30° C (59° and 86° F) permitted. Reconstituted cangrelor should be further diluted immediately. Cangrelor is stable at RT for 12 hours diluted in D5W and for 24 hours diluted in NS. Discard any unused portion of reconstituted solution remaining in the vial.
Compatibility
Manufacturer states, “Administer cangrelor via a dedicated IV line.”
Rate of administration
Administer cangrelor via a dedicated IV line.
Bolus:
Administer the bolus volume rapidly (less than 1 minute) from the diluted bag via manual IV push or pump. Ensure the bolus is completely administered before the start of PCI. Start the infusion immediately after administration of the bolus.
Infusion:
Administer at 4 mcg/kg/min. Continue for at least 2 hours or for the duration of PCI, whichever is longer.
Actions
A direct P2Y12 platelet receptor inhibitor that blocks adenosine diphosphate (ADP)–induced platelet activation and aggregation. It binds selectively and reversibly to the P2Y12 receptor to prevent further signaling and platelet activation. When given according to the recommended regimen, platelet inhibition occurs within 2 minutes. Metabolism of cangrelor is independent of hepatic function and does not interfere with other drugs metabolized by hepatic enzymes. It is deactivated rapidly in the circulation by dephosphorylation to its primary metabolite, a nucleoside that has negligible antiplatelet activity. After discontinuation of the infusion, the antiplatelet effect decreases rapidly and platelet function returns to normal within 1 hour. The average elimination half-life is about 3 to 6 minutes. Excreted in urine and feces.
Indications and uses
An adjunct to percutaneous coronary intervention (PCI) to reduce the risk of periprocedural myocardial infarction (MI), repeat coronary revascularization, and stent thrombosis (ST) in patients who have not been treated with a P2Y12 platelet inhibitor and are not being given a glycoprotein IIb/IIIa inhibitor.
Contraindications
Patients with significant active bleeding. ■ Known hypersensitivity (e.g., anaphylaxis) to cangrelor or any component of the product.
Precautions
For IV use only. ■ Bleeding is the most common complication encountered during therapy. ■ Drugs that inhibit platelet P2Y12 function, including cangrelor, increase the risk of bleeding. ■ Once cangrelor is discontinued, there is no antiplatelet effect after 1 hour. ■ Serious hypersensitivity reactions have been reported.
Monitor:
Monitor for S/S of bleeding. ■ Monitor for S/S of hypersensitivity reactions (e.g., anaphylaxis, hypotension, pruritus, rash, urticaria, or wheezing).
Patient education:
Promptly report S/S of a hypersensitivity reaction (e.g., hives, rash, shortness of breath or troubled breathing, swelling of eyelids, lips, or face).
Maternal/child:
Category C: use during pregnancy only if clearly needed. ■ It is not known if cangrelor is excreted in human milk. ■ Safety and effectiveness for use in pediatric patients not established.
Elderly:
No overall difference in safety or effectiveness between older and younger patients has been observed.
Drug/lab interactions
If clopidogrel or prasugrel is administered during cangrelor infusion, it will have no antiplatelet effect until the next dose is administered. Therefore clopidogrel and prasugrel should not be administered until cangrelor infusion is discontinued. ■ Administration of ticagrelor during cangrelor infusion does not attenuate the antiplatelet effect of ticagrelor. ■ Coadministration of cangrelor with unfractionated heparin, aspirin, and nitroglycerin was formally studied in healthy subjects, with no evidence of an effect on the pharmacokinetics and pharmacodynamics of cangrelor. ■ Cangrelor has been coadministered with bivalirudin, low-molecular-weight heparin, clopidogrel, prasugrel, and ticagrelor without clinically detectable interactions.
Side effects
The most common adverse reaction is bleeding.
Coronary artery dissection, coronary artery perforation, and dyspnea were the most frequent events leading to discontinuation of cangrelor. Serious cases of hypersensitivity, including angioedema, anaphylactic reactions, anaphylactic shock, bronchospasm, and stridor, have occurred. Decreased renal function was reported in a small number of patients with severe renal impairment (creatinine clearance less than 30 mL/min).
Antidote
There is no specific treatment to reverse the antiplatelet effect of cangrelor, but the effect is gone within 1 hour after discontinuation of the drug. Treat anaphylaxis immediately with oxygen, epinephrine (Adrenalin), antihistamines (e.g., diphenhydramine [Benadryl]), vasopressors (e.g., dopamine), corticosteroids, albuterol, IV fluids, and ventilation equipment as indicated. Resuscitate as necessary.
Capreomycin 
(kap-ree-oh-MYE-sin)
Capastat
Antibacterial (antituberculosis)
Usual dose
1 Gm daily, not to exceed 20 mg/kg/day. Give for 60 to 120 days, followed by 1 Gm two or three times weekly. Therapy for tuberculosis should be maintained for 12 to 24 months. Administered in combination with at least one other antituberculosis agent to which the patient’s strain of tubercle bacilli is susceptible.
Dose adjustments
The elderly and patients with reduced renal function should have dosage reduction based on CrCl using the guidelines in the following chart. These dosages are designed to achieve a mean steady-state capreomycin level of 10 mcg/mL. Elevation of BUN above 30 mg/dL or any other evidence of decreasing renal function warrants evaluation of patient. Dose reduction or discontinuation of therapy may be required.
| Estimated Dosages to Attain Mean Steady-State Serum Capreomycin Concentration of 10 mcg/mL Based on CrCl | |||
| CrCl (mL/min) | Dose* (mg/kg) Based on Dosing Intervals | ||
| 24 hr | 48 hr | 72 hr | |
| 0 mL/min | 1.29 mg/kg | 2.58 mg/kg | 3.87 mg/kg |
| 10 mL/min | 2.43 mg/kg | 4.87 mg/kg | 7.30 mg/kg |
| 20 mL/min | 3.58 mg/kg | 7.16 mg/kg | 10.7 mg/kg |
| 30 mL/min | 4.72 mg/kg | 9.45 mg/kg | 14.2 mg/kg |
| 40 mL/min | 5.87 mg/kg | 11.7 mg/kg | |
| 50 mL/min | 7.01 mg/kg | 14.0 mg/kg | |
| 60 mL/min | 8.16 mg/kg | ||
| 80 mL/min | 10.4 mg/kg† | ||
| 100 mL/min | 12.7 mg/kg† | ||
| 110 mL/min | 13.9 mg/kg† | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


