18 Toxicology and Poisonings
Pearls
• The “ABCDE” primary assessment (Airway, Breathing, Circulation, Disability, Exposure) similar to that used to evaluate and stabilize trauma victims, is a useful tool to assess and stabilize poisoned patients.
• During stabilization of the patient with poisoning or overdose, general principles of pediatric advanced life support (PALS) apply. Focus should begin with support of airway, oxygenation, ventilation and circulation.
• Advanced life support for all poisoned patients includes meticulous attention to maintaining a patent airway and adequate oxygenation, ventilation, and circulation. Children with overdoses of some drugs may require modified resuscitation therapies or sequences.
• The critical care nurse should carefully analyze the ECG for changes that may be caused by tricyclic antidepressants (TCAs), calcium channel blockers, and beta (β)-blockers. Such changes include a widened QRS complex, prolonged corrected QT interval (QTc), bradycardia, sino-atrial (SA) and atrioventricular (AV) nodal conduction delays, ventricular tachycardia (VT), ventricular fibrillation (VF), and asystole.
• Adjustments in the bolus volume used for fluid resuscitation may be necessary for children who ingest drugs that affect myocardial contractility or drugs that may contribute to the development of noncardiogenic pulmonary edema. In these patients boluses of 5 to 10 or 10 to 15 mL/kg may be used instead of the traditional 20 mL/kg bolus. In general, fluid boluses can be administered over 5 to 20 minutes, but when myocardial contractility is compromised or pulmonary edema is present, the bolus is typically administered over about 10 to 20 minutes. Reassess the patient carefully between boluses, be prepared to support oxygenation and ventilation (with possible continuous positive airway pressure), and repeat the bolus as needed.
Scope of the problem
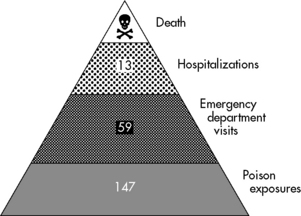
Poisonings and toxic exposures resulting in injury or death are significant problems for pediatric emergency and critical care. In 2009, approximately 1.6 million poisonings occurred in children 19 years of age or younger.26 From 1995 to 2005, poisoning accounted for 1.2 million emergency department visits.120 The average annual rate of poisoning-related visits was disproportionately higher among children under 5 years of age than among children in older age categories.107 Poisonings and drug overdoses are the most common toxicities that result in admission to pediatric critical care units.107 Figure 18-1 illustrates the burden of poisoning in the United States.
Toxic exposure can complicate resuscitation priorities and support. In unusual cases of poisoning or when life-threatening complications are anticipated, the American College of Emergency Physicians (ACEP) and American Academy of Clinical Toxicology (AACT) recommend consultation with a medical toxicologist or certified regional poison information center and transfer to a poison treatment center.2,7 Dedicated poison treatment centers can provide diagnostic and treatment services beyond those available in most hospitals. Poisoned children with life-threatening complications should ideally receive care at a children’s hospital or Emergency Department Approved for Pediatrics (EDAP) facility.
This chapter provides an overview of the general approach to the poisoned patient. It highlights the epidemiology, clinical recognition, and management of five major types of poisonings and overdose: cocaine, calcium channel blockers, β-adrenergic blockers, opioids, and TCAs. It is consistent with the detailed recommendations contained in the Toxicology chapter of the American Heart Association (AHA) 2002 Pediatric Advanced Life Support (PALS) Provider Manual,61 developed by Scalzo, Hazinski, et al. In addition, the recommendations are consistent with those in the Pediatric Advanced Life Support section of the 2010 AHA Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care.79
General approach to the poisoned patient
Life-threatening morbidity associated with poisoning may manifest as respiratory depression, seizures, depressed level of consciousness, hypotension and cardiac arrhythmias. The ten top exposures causing death are listed in Box 18-1.
Box 18-1 Ten Top Exposures Causing Deaths in 2009
• Sedatives/hypnotics/antipsychotics
• Cardiovascular drugs (i.e., calcium channel blockers and β-blockers)
• Acetaminophen in combination with narcotic analgesics (e.g., opioids)
• Stimulants and street drugs (e.g., cocaine)
From Bronstein AC, et al: 2009 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 27th annual report. Clin Toxicol 48:979-1178, 2010.
In addition to a thorough history and assessment of drugs and toxic chemicals in the child’s environment, physical findings beyond those detected by the primary and secondary assessments may have particular value and diagnostic significance for the patient with a toxic exposure (Box 18-2). The characteristic clinical manifestations of a specific poisoning or toxin are termed a toxidrome. Examples of toxidromes include: (1) the clinical constellation of tachycardia, mydriasis, diaphoresis, seizures, and the presence of bowel sounds suggests the sympathomimetic toxidrome (e.g., cocaine); (2) the combination of tachycardia, mydriasis, dry skin, seizures, and absent bowel sounds is consistent with the anticholinergic toxidrome (e.g., atropine).61 Recognition of common toxidromes can be vital to efficient resuscitation, supportive care, decontamination, and administration of antidotes.
Regardless of the type of toxin, protection of the airway is of fundamental importance in the management of any poisoning. Elective endotracheal intubation in a poisoned patient should be considered earlier than in a patient without a history of toxin exposure because poisoned patients are at high risk for development of sudden or progressive respiratory failure. Many toxins cause respiratory failure by depression of respiratory drive, hypoperfusion of the central nervous system (CNS), or direct toxic effects on the CNS or pulmonary systems. Toxins can affect oxygenation by causing alveolar hypoventilation (e.g., opiate intoxication)54 or direct pulmonary toxicity (e.g., TCAs).41 Table 18-1 lists potential mechanisms and toxic agents that cause decreased oxygenation in the poisoned patient.
Table 18-1 Potential Mechanisms of Decreased Oxygenation in Poisoning
| Mechanism | Examples of Toxic Etiology |
| Alveolar hypoventilation (hypercarbia with normal alveolar-arterial oxygen difference) | Opiates, tricyclic antidepressants, benzodiazepines, barbiturates, and clonidine |
| Ventilation-perfusion mismatch (includes acute respiratory distress syndrome) | Direct pulmonary toxicity (e.g., tricyclic antidepressants, calcium channel blockers, or inhaled hydrocarbons) or secondary injury resulting from decreased level of consciousness and aspiration of gastric contents, or pulmonary embolism (complication of chronic IV drug abuse) |
| Intrapulmonary shunting | Pneumothorax caused by cocaine, by internal jugular injections of heroin by drug abusers, and by iron intoxication |
| Diffusion abnormality | Chlorine, chloramine, and ammonia gas inhalation |
| Decrease in alveolar oxygen content | Simple asphyxiants: carbon dioxide, methane, inhalants (e.g., propane, butane, fluorocarbons), and nitrogen oxides |
Modified from Hazinski MF, et al: Toxicology. In PALS provider manual. Dallas, 2002, American Heart Association, p. 307.
Nondepolarizing neuromuscular blocking agents, such as vecuronium and rocuronium, are most useful for the intubation of poisoned patients. They have minimal cardiovascular side effects, rapid onset, and a relatively short duration of action (see also Chapter 5). The use of short-acting sedatives, in conjunction with short-acting neuromuscular blocking agents, enables rapid and efficient repeat assessment of a patient’s mental status. Such assessments are particularly important in the management of patients with status epilepticus.61
If the poisoned patient demonstrates effective spontaneous breathing and can maintain airway patency, consider placing the patient in a recovery position. The left lateral decubitus position reduces absorption of ingested substances154 as well as the risk for aspiration.
Gastrointestinal Decontamination
If a patent airway can be maintained in a poisoned patient, gastrointestinal decontamination may be considered. It is important to note that gastrointestinal decontamination has not been shown to improve outcome35,153 as defined by morbidity, mortality, cost, or length of hospital stay.84 In addition to achieving skill in administering gastrointestinal decontamination, critical care nurses should understand the inherent risks and benefits.
Administration of oral fluids for dilution purposes is of no proven benefit in most poisonings. In instances of acid ingestion, limited animal data suggests that oral dilution with water or milk as a demulcent may be helpful.67
Administration of syrup of ipecac is not recommended by the American Academy of Pediatrics for the treatment of poisonings.5,84 When applicable, recommended gastric decontamination basically consists of activated charcoal and rarely gastric lavage.
Activated Charcoal
Activated charcoal adsorbs many drugs as well as some other compounds.38 Although there is no evidence that administration of activated charcoal improves clinical outcome,35 it is often considered when children present to emergency care very soon after toxic ingestion. Caution is advised to limit the use of activated charcoal in children less than 6 months of age because they have a high risk of aspiration. Activated charcoal reduces the mean bioavailability of the drugs by approximately 69% when it is given within 30 minutes after drug or toxin ingestion; however, the bioavailability of drugs is only reduced by half that amount when activated charcoal is given an hour or more after ingestion.34,35
Most toxicologists and poison centers do not recommend prehospital administration of activated charcoal, although emergency department administration of activated charcoal may be useful in the treatment of some poisonings, particularly if the ingestion has occurred within 1 hour of presentation. There are insufficient data to either support or exclude the use of activated charcoal at greater than 1 hour after an ingestion.34,35
The optimal dose of activated charcoal has not been established in controlled human trials. The AACT and the European Association of Poisons Centres and Clinical Toxicologists have developed consensus oral dose recommendations for activated charcoal (Box 18-3).34,35
Repeated doses of activated charcoal can be administered to treat certain specific ingestions,3 but there is no evidence that multi-dose administration is superior to a single dose.45 Use of multiple dose activated charcoal is not recommended if the toxic agent slows gastrointestinal motility (e.g., TCAs, calcium channel blockers, and opiates), because the activated charcoal can contribute to regurgitation and aspiration or can become impacted, leading to intestinal perforation.56
Contraindications to the administration of activated charcoal include an unprotected airway, ingestion of volatile substances (e.g., hydrocarbons) and anatomic anomalies of the gastrointestinal tract. Administration of activated charcoal may lead to regurgitation and aspiration, hence placement of an endotracheal tube before administration of activated charcoal may reduce but not reliably prevent aspiration.114
Gastric Lavage
Although emergency personnel have used gastric lavage for years, there is no convincing evidence that it improves clinical outcome.153 Like activated charcoal, gastric lavage increases the risk of aspiration.78,138,152 In addition, complications of lavage tube placement include hypoxia,151 tension pneumothorax and charcoal-containing empyema,71 and esophageal9 and gastrointestinal perforation.102 For these reasons, gastric lavage is only indicated in a patient who presents soon after ingestion of some life-threatening toxins (Box 18-4).
Box 18-4 Key Issues in Gastrointestinal Decontamination
• Sudden changes in level of consciousness and respiratory depression may develop in poisoned pediatric patients. For this reason, support the ABCs and establish and maintain a patent airway before administering gastrointestinal decontamination. Alert patients may be able to maintain their airways, whereas others will require intubation.
• Syrup of ipecac is not recommended for any of the five types of poisoning discussed in this chapter.
• If activated charcoal is indicated, administer it early after ingestion because it is most effective within 1 h of ingestion.
• Gastric lavage is not a routine intervention; its effectiveness is limited in poisoned patients. Lavage may be considered for asymptomatic patients who present early (usually within 60 min or less) after ingestion of some life-threatening toxins.
• Whole bowel irrigation may be beneficial in select poisonings, but further research is needed.
Antidotes
Following the assessment and support of airway, oxygenation, ventilation, and circulation, the critical care nurse may need to administer a specific antidotal therapy. Use of true antidotes as defined by the International Programme for Chemical Safety (IPCS) is relatively infrequent in pediatric poisonings, with the exception of naloxone, which is a classic antidote that effectively reverses opiate toxicity. The IPCS classifies naloxone as an A1 agent (i.e., A: should be available within 30 min or less; 1: effectiveness is well documented).70,132 A list of other antidotes is included in Table 18-2.
Table 18-2 Common Antidotes for Common Poisons
| Poison | Antidote(s) |
| Acetaminophen | N-acetylcysteine (NAC) |
| Organophosphate and Carbamate Insecticides, Nerve Agents | Atropine Pralidoxime (2-PAM); (not usually required with Carbamate Insecticides) |
| Iron | Deferoxamine |
| Digoxin | Digoxin Immune Fab |
| Mercury | Dimercaprol (BAL) |
| Benzodiazepines | Flumazenil (not recommended for overdose but may be used to reverse procedural sedation) |
| Methanol, ethylene glycol | Fomepizole |
| Cyanide | Hydroxocobalamin (preferred) Cyanide Antidote Package |
| Opioids | Naloxone |
| Lead | Succimer (DMSA) |
Management of specific poisonings
Cocaine
Epidemiology, Pathophysiology, and Clinical Manifestations
Cocaine has complex pharmacologic effects, and the route of administration and the form of cocaine involved can affect the onset, duration, and magnitude of the clinical signs and symptoms and potential complications.14 Cocaine is absorbed from all mucous membranes, from the gastrointestinal tract (most common route in pediatric unintentional exposure), and the genitourinary tract.55,61
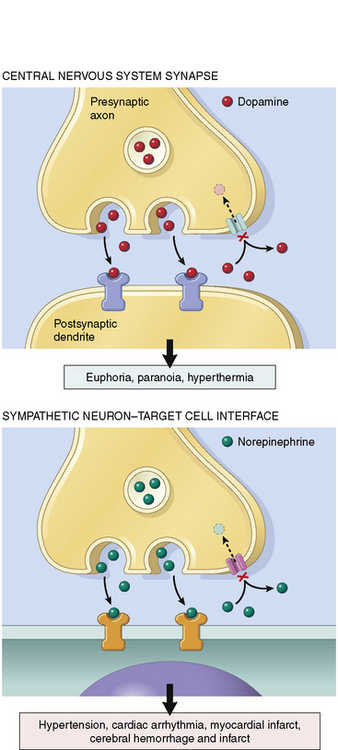
Cocaine binds to the reuptake pump in presynaptic nerves, blocking the uptake of norepinephrine, dopamine, epinephrine, and serotonin from the synaptic cleft. This action leads to local accumulation of these neurotransmitters (Fig. 18-2), which produces both peripheral and CNS effects.61
The accumulation of norepinephrine and epinephrine at β-adrenergic receptors results in tachycardia, increased myocardial contractility, tremor, diaphoresis, and mydriasis. Tachycardia increases myocardial oxygen demand while reducing the time for diastolic filling and for coronary perfusion (particularly of the left ventricle).87 Accumulation of neurotransmitters at peripheral α-adrenergic receptors results in vasoconstriction and hypertension. The peripheral endothelial nitric oxide system can also be impaired, leading to further vasoconstriction.113
Centrally mediated dopaminergic effects of cocaine include mood elevation and movement disorders. Centrally mediated stimulation of serotonin (i.e., 5-hydroxytryptamine or 5-HT) receptors results in exhilaration, hallucinations, and hyperthermia. Stimulation of peripheral 5-HT receptors also results in coronary artery vasospasm that can lead to acute coronary syndrome (ACS) and myocardial infarction. In addition, cocaine stimulates both platelet aggregation62 and increases in circulating epinephrine; these effects can lead to secondary platelet activation and coronary occlusion.73
In adults, the most frequent cause of cocaine-induced hospitalization is ACS, caused by coronary vasoconstriction and platelet aggregation with resulting myocardial ischemia, chest discomfort and possible infarction.24,65,87 Although ACS is a rare complication in children, it has been reported, particularly when ethanol and cocaine are combined.164 Concurrent use of cocaine and ethanol precipitates the formation of the cocaine metabolite, cocaethylene, which increases the cardiotoxic and neurotoxic effects of either substance alone.48 Although myocardial infarction in the neonate with a structurally normal heart and coronary arteries is rare, its association with maternal cocaine abuse has been reported.28
Cocaine-induced ACS can lead to myocardial ischemia and subsequent infarction and complications such as ventricular arrhythmias, congestive heart failure, and death.61,65 Cocaine-induced ACS is diagnosed by ECG changes characteristic of myocardial ischemia; infarction has occurred if serum troponin levels are elevated. In addition to ischemia-induced arrhythmias, cocaine also disturbs cardiac electrophysiology by altering sodium and potassium channel conduction and may induce wide-complex arrhythmias, VT, and VF, including torsades de pointes.13,14,81,127
Infants can be exposed to cocaine in breast milk,166 and infants or children may experience passive inhalation of vapors from adults smoking crack cocaine.63,116 The presence of the cocaine metabolite benzoylecgonine in the urine of children who are otherwise medically stable may reflect passive inhalation; it does not necessarily indicate poisoning or intentional cocaine administration.63 This situation raises legitimate concern for the well-being of such children, because deaths have been linked to passive inhalation of crack cocaine smoke.112
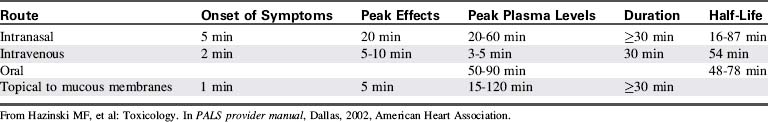
Because cocaine is rapidly metabolized, serum levels generally are of little use and often do not correlate with clinical findings.19 Table 18-3 summarizes the pharmacokinetics and pharmacodynamics of cocaine hydrochloride.
Management
General Care
Initial treatment of cocaine toxicity consists of oxygen administration, continuous ECG monitoring, and administration of a benzodiazepine (e.g., diazepam or lorazepam).42,61 This care is summarized in Box 18-5.
Box 18-5 Recognition and Management of Cocaine Toxicity
Management
Provide continuous ECG monitoring; observe for and treat ventricular arrhythmias (for arrhythmias secondary to infarction, consider lidocaine).
Treat hyperthermia aggressively.
For ACS, consider nitroglycerin, a benzodiazepine, and phentolamine.
Consider administration of sodium bicarbonate (1-2 mEq/kg).
Benzodiazepine administration is the mainstay of cocaine toxicity treatment because it offers both anticonvulsant and CNS-depressant effects, and it reduces heart rate and systemic arterial pressure.42 Benzodiazepines also appear to attenuate the toxic myocardial effects of cocaine.87 In contrast, phenothiazines and butyrophenones (e.g., haloperidol) provide no benefit and may be harmful to patients with cocaine toxidrome. β-Blockers are contraindicated in cocaine intoxication because their use has been associated with increased blood pressure, coronary vasospasm and fatal myocardial infarction.79
Because cocaine is a sodium channel blocker, sodium bicarbonate in a dose of 1 to 2 mEq/kg may be effective in the treatment of cocaine-associated ventricular arrhythmias.61,79 In an experimental model of cocaine-induced ECG changes, sodium bicarbonate significantly reduced the prolonged PR and QT intervals and reduced the QRS duration.127 Sodium bicarbonate also may be effective in treating the cocaine-associated acidemia that is thought to contribute to intraventricular conduction delays (prolonged QRS interval), arrhythmias, and depressed myocardial contractility.156
Lidocaine administration may be considered for patients with ventricular arrhythmias associated with cocaine-induced myocardial infarction that are refractory to other treatments.79 The effectiveness of lidocaine in this patient population has not been well established. Because of the fact that lidocaine inhibits fast sodium channels, it has been shown to potentiate cocaine toxicity in animals,43 although this effect has not been documented in humans. Cocaine and lidocaine together also may have additive effects that increase the likelihood of seizure activity.142,168
The effectiveness of epinephrine in the treatment of cocaine-induced circulatory failure is questionable.111 Epinephrine may exacerbate cocaine-induced arrhythmias and should not be administered for ventricular arrhythmias. If VF or pulseless VT develop, epinephrine is used to increase coronary perfusion pressure during cardiopulmonary resuscitation (CPR).61
Treatment of Hyperthermia
The CNS manifestations of cocaine intoxication often include loss of thermoregulation with resulting hyperthermia. High ambient temperature has been associated with a significant increase in mortality from cocaine overdose in humans.104,105 As a result, vigilant monitoring of body temperature is indicated for all patients with cocaine intoxication, and fever should be treated aggressively.79 External cooling is necessary for children presenting with agitation, delirium, seizures, and elevated body temperature.
Treatment of Seizures
Cocaine may produce seizures in infants and children after ingestion,37,46 and in infants when the drug is transmitted through breast milk.33 Cocaine likely causes seizures by affecting gamma aminobutyric acid (GABA) transmission; it also may stimulate the neuroexcitatory N-methyl D-aspartate (NMDA) receptor. Seizure management includes administration of a benzodiazepine. Lorazepam is often used (0.05-0.1 mg/kg, up to 2 mg/dose), with doses repeated as needed. Following administration of benzodiazepines, particularly when repeated doses are necessary (e.g., to manage prolonged cocaine-induced seizures), patients should be closely monitored for development of respiratory depression. Phenytoin and fosphenytoin may not be effective in treating cocaine-induced seizures because they lack an effect on the GABAA receptor.159 Phenobarbital is recommended for the treatment of seizures refractory to benzodiazepines. Propofol also may be of benefit to control cocaine-induced seizures because it has a short half-life, making it easy to titrate according to patient response.159
Calcium Channel Blocker Toxicity
Epidemiology, Pathophysiology, and Clinical Manifestations
The increasing use of calcium channel blockers for the treatment of hypertension and congestive heart failure makes them readily available for unintentional or intentional overdose. In 2009, a total of 10,868 exposures to calcium channel blockers were reported to the AAPCC; nearly 14% of these exposures occurred in children younger than 6 years.26
Although calcium channel blockers can be classified according to their effects on the myocardium and vascular smooth muscle, in cases of overdose these selective properties are lost and serious cardiovascular toxicity may be seen with all agents.134 All calcium channel blockers bind to calcium channels, inhibiting the influx of calcium into cells. As a result, these agents will affect impulse conduction in slow-channel-dependent tissue such as the sinoatrial (SA) and AV nodes, coupling of myocardial excitation-contraction, and vascular smooth muscle tone.
The life-threatening clinical manifestations of calcium channel toxicity include bradyarrhythmias (caused by inhibition of pacemaker cells) and hypotension (caused by vasodilation and impaired cardiac contractility).115,134 Electrocardiographic changes can include a prolonged PR interval, inverted P waves, AV dissociation, AV block,1 ST segment changes, low-amplitude T waves, sinus arrest, and asystole. Cerebral hypoperfusion can cause altered mental status (e.g., syncope, seizures, and coma).
The lung and gastrointestinal system are affected directly or indirectly by calcium channel blocker poisoning. Pulmonary complications include cardiogenic and noncardiogenic pulmonary edema,69,90 which will necessitate cautious fluid resuscitation and early support of ventilation.
Gastrointestinal complications include hypomotility, ileus,49 and constipation; these effects may be secondary to the inhibition of gastrointestinal motility hormone release.135 Patients with calcium channel blocker overdose often have absent or greatly diminished bowel sounds. Use of activated charcoal or whole-bowel irrigation may not be appropriate for these patients.
Careful serial assessment of bowel sounds should be performed if any form of gastrointestinal decontamination is being considered, particularly if the patient has ingested sustained-release products. Some experts advocate whole-bowel irrigation for patients who ingest sustained-release products to prevent further absorption,148 but controlled trials have not been performed to determine the effectiveness of whole bowel irrigation after calcium channel blocker overdose.4
Management
General Care
Although the supportive and specific therapies discussed in this section can be very effective in children, third-degree atrioventricular (AV) block with cardiac arrest161 and death have been reported.39,89 As a result, providers should monitor the patient closely and be prepared to institute resuscitation. Onset of symptoms may be immediate or delayed for up to 12 to 16 hours, especially when a sustained-release preparation has been ingested.145,157
The initial approach to therapy for calcium channel blocker overdose is to support oxygenation and ventilation, provide continuous ECG monitoring, and carefully monitor and support cardiovascular function and systemic perfusion (Box 18-6). All patients with a significant overdose require close monitoring of blood pressure because severe myocardial dysfunction and hypotension may develop. Continuous intra-arterial blood pressure monitoring should be considered for symptomatic patients.