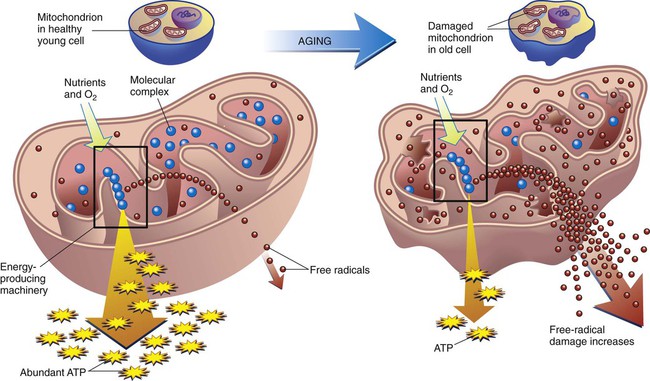
On completion of this chapter, the reader will be able to: 1. Describe the interrelationships between the various biological theories of aging. 2. Compare and contrast theories of programmed aging with error theories of aging. 3. Recommend strategies to promote healthy aging that are consistent with the state of the science of biological theories of aging. 4. Compare and contrast the major psychosocial theories of aging. 5. Use at least one sociological theory of aging to support or refute commonly provided social services for older adults living in the community. 6. Create strategies to foster the highest level of wellness in aging, based on the developmental theories of aging. 7. Explain how personality may influence the lived experience of aging. In everyday language, aging is most often described in terms of chronology, or by the measurement of time since birth. However, each culture has its own definition of when one becomes recognized as a “senior” or elder or older adult. Some groups define aging in functional terms; that one becomes “old” when one is no longer able to perform one’s usual activities (Jett, 2003). Social aging is determined by role, such as retiree or as a wise woman/man of the clan; of status change when a parent becomes a grandparent. Transitions into the status of “senior” are often marked by ritual, such as special celebrations, invitations to join groups such as the American Association of Retired Persons or the qualification for “senior discounts.” Although this number is rising, one qualifies for “old age” benefits in the United States at 65 years of age. Physiological aging is a complex process involving every cell in the body. The physical traits by which we identify one as older (e.g., gray hair, wrinkled skin) are referred to as the aging phenotype, that is, an outward expression of one’s individual genetic makeup (Carnes et al., 2008). Biological aging, referred to as senescence, is an exceedingly complex, genetically regulated, interactive process of change (Ostojić et al., 2009). Whereas there is a growing body of knowledge about the genomics of aging, what triggers the associated changes at the cellular or organ level is still a topic of debate. However, it is commonly believed that the aging phenotype reflects declining functional capacity of the most basic structures in the cells, which in turn affects the functioning of the organism, be it a yeast cell, a mouse, or a human (National Institute of Aging [NIA], 2003). Is the timing of the decline preprogrammed within the cell itself, or is it random? Is there a maximal life span? How important is the lifetime effect of the environment—be it physical (e.g., pollution) psychological, or social (e.g.inequity)? Why do some people age faster than others? (Box 3-1). And perhaps most importantly of all, what is the relationship between aging and illness? If this were true, the organism would not change; it would not “age.” Instead, the cells become increasingly complex. For example, an infant does not learn to walk or talk until the neurons have adequate myelination, that is, until the myelin sheath is thick enough to facilitate smooth and rapid transmission of impulses. The cells also accumulate damage resulting in errors seen in replication. These changes are made visible in the traits we associate with aging. The association of cellular errors and the aging phenotype is no longer in question (Nomellini et al., 2008). It is the causation and patterns of effect that are in debate and discovery. We do not know for certain if the damage and changes are orderly and predictable, or random and chaotic (Box 3-2). Programmed aging theories suggest that aging is the result of predictable cellular death; that the cell and organism have genetically determined life spans referred to as the Hayflick limit or the biological clock (NIA, 2003). Much of the contemporary work on programmed aging has evolved from the groundbreaking work of Hayflick and Moorehead (1981). In a test of their theory, they found that living cells could be preserved indefinitely by keeping them at subzero temperatures. However, when thawed, the cells continued to replicate from the point at which they had been interrupted, and the total number of replications remained constant. The neuroendocrine control or pacemaker theory explains aging as a programmed decline in the functioning of the nervous, endocrine, and immune systems (De la Fuente, 2008). It is not that the cells “die”; instead, they lose their ability to reproduce, a process referred to as replicative senescence. Current research centers on the effect of hormones on neuroendocrine functioning, especially dehydroepiandrosterone (DHEA) and melatonin. DHEA is produced by the adrenal glands, and the amount secreted diminishes with time. Adding DHEA to the diets of experimental animals appears to have increased their longevity and bolstered their immune response (Legrain and Girard, 2003; Perrini et al., 2005). Melatonin, produced by the pineal gland, has been found to be a powerful regulator of biological rhythms and antioxidants. Its secretion is also markedly reduced as one ages (Srinivasan et al., 2005). Other hormones under examination in relation to aging are growth factors, estrogen, and testosterone (NIA, 2003). The immune system in the human body is a complex network of cells, tissues, and organs that function separately, but together protect the body from invasion by exogenous substances, such as pathogens. The body maintains homeostasis through the actions of this protective, self-regulatory system, controlled by B lymphocytes (humoral immunity) and T lymphocytes (cellular immunity) (De la Fuente, 2008). The immunity theory presents aging as a programmed accumulation of damage and decline in the function of the immune system, or immunosenescence. The damage is the result of oxidative stress (see p. 36). The ability of lymphocytes to withstand oxidative stress appears to be a key factor in the aging phenotype; T lymphocytes show more signs of “aging” than do B lymphocytes (Swain and Nikolich-Zugich, 2009). The T cells are thought to be responsible for hastening the age-related changes caused by autoimmune reactions as the body battles itself. Cellular errors in the immune system have been found to lead to an auto-aggressive phenomenon in which normal cells are misidentified as alien and are destroyed by the body’s own immune system. This phenomenon is used to explain the increase in autoimmune disorders as we age (De la Fuente, 2008). An increasing number of health problems common in later life are being considered in terms of impaired immunosenescence and autoimmunity. Alzheimer’s disease, rheumatoid conditions, atherosclerosis, hypertension, and thromboembolism are among those that have been studied in association with immunity and directly linked to related oxidative stress (see (Box 3-3). Of significant importance in the clinical setting is the increased susceptibility to infections, autoimmune disorders, and cancers (Gomez et al., 2008). One of the earliest theories of aging, the wear-and-tear theory, proposes that cell errors are the result of “wearing out” over time because of continued use. Cells are aggravated by the harmful effects of internal and external stressors, which include pollutants and injurious metabolic by-products, now known as free radicals. These may cause a progressive decline in cellular function or the death of an increasing number of cells. Striated muscle, heart muscle, muscle fibers, nerve cells, and the brain are irreplaceable when destroyed by wear and tear or by mechanical or chemical injury (Carnes et al., 2008). As science advanced so did the understanding of the ideas proposed in the wear-and-tear theory of aging. The cross-linkage theory describes aging as the result of accumulated damage from errors associated with cross-linked proteins. For unknown reasons cellular glucose attaches to protein, either by glycosylation or by glycation. Once attached, a chain reaction of bonding or “cross-linking” between the protein and the glucose causing them to become stiff and thick. The newly cross-linked proteins are called AGEs, or advanced glycation end-products (Eyetsemitan, 2007). Cross-linking is an area of research especially as it applies to wound healing. Because collagens are the most plentiful proteins in the body, this is where the cross-linking can be seen most easily in the form of stiffened joints and skin. Skin that was once smooth, silky, firm, and soft becomes dry, sags, and is less elastic. Collagen is also a key component of the lungs, arteries, and tendons. Similar changes can be seen there (see Chapter 4). Cross-linking may also cause cholesterol to attach to cell walls, leading to atherosclerosis, and the lens to cloud and stiffen, leading to cataracts. Substances that may act as cross-linking agents include unsaturated fats and metal ions such as aluminum, zinc, and magnesium. Many medications taken by older adults (e.g., antacids, anticoagulants) contain aluminum and may exacerbate cross-linking, although a causative effect has not yet been clarified (Valko et al., 2005). The free radical or oxidative stress theory of aging has garnered strong support as correlative evidence has accumulated over the last 35 years (Jang and Van Remmen, 2009). Proponents of this theory postulate that the errors are the result of random damage from free radicals. Free radicals are molecules that contain unpaired ions and therefore an extra electrical charge. They exist only momentarily but are highly reactive to molecules in the cell membrane. As the free ion charge latches onto other molecules, damage occurs, especially to the membranes of unsaturated lipids such as mitochondria. Free radicals are natural by-products of the cellular metabolism of oxygen and are always present to some extent. In the immune system they are useful for destroying bacteria and other foreign substances. The accumulation of free radicals is referred to as “oxidative stress” or “oxidative damage.” Mitochondrial DNA appears to be most affected by these changes (Figure 3-2) (Gruber et al., 2008).
Theories of Aging
![]() http://evolve.elsevier.com/Ebersole/TwdHlthAging
http://evolve.elsevier.com/Ebersole/TwdHlthAging
What is Aging?
Biological Theories of Aging
Cellular Functioning
Programmed Aging Theories
Neuroendocrine Control or Pacemaker Theory
Immunity Theory
Error Theories
Wear-and-Tear Theory
Cross-linkage Theory
Oxidative Stress Theory (Free Radical Theory)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Theories of Aging
Get Clinical Tree app for offline access

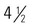 months, a supercentenarian. In 1965, when she was 90 years old, her lawyer recognized the value of the apartment in which she lived and owned and made her, what turned out to be, the deal of a lifetime. In exchange for the deed to the apartment, he would pay her a monthly “pension” for life and she could live in the apartment the rest of her life. Over the next 32 years she was paid three times the apartment’s value. She also outlived the lawyer, his son, her husband of 50 years, her daughter, and her only grandson. An active woman, she took up fencing at 85 and was still riding a bike at 100. She smoked until she was 117 and preferred a diet rich in olive oil (
months, a supercentenarian. In 1965, when she was 90 years old, her lawyer recognized the value of the apartment in which she lived and owned and made her, what turned out to be, the deal of a lifetime. In exchange for the deed to the apartment, he would pay her a monthly “pension” for life and she could live in the apartment the rest of her life. Over the next 32 years she was paid three times the apartment’s value. She also outlived the lawyer, his son, her husband of 50 years, her daughter, and her only grandson. An active woman, she took up fencing at 85 and was still riding a bike at 100. She smoked until she was 117 and preferred a diet rich in olive oil (