Pulse 105 bpm
Sinus rhythm
Temperature 38.5°C
Respiratory rate 18 bpm
SpO2 97%
PaO2 11 kPa
PaCO2 5.2 kPa
HCO3− 20 mmol/L
Base excess –4
Oral intake 400 mL since midnight (8 h)
Intravenous input 150 mL (IV antibiotics)
Urea 18 mmol/L
Potassium 5 mmol/L
Sodium 146 mmol/L
Hb 12.5 g/dL
WCC 15 000
CRP 12 mg/L
Lactate 2.3 mmol/L
Scenario
Elizabeth James is a 48-year-old schoolteacher admitted to HDU 12 hours ago with community-acquired pneumonia. Past medical history includes regular migraines for which she takes ibuprofen. Despite 12 hours of intravenous (IV) antibiotics, her condition is deteriorating. Significant features of her clinical presentation can be seen in Table 9.1.
Reader activities
Having read this scenario, consider the following:
- What aspects of Elizabeth’s assessment would make you think she has AKI?
- What do you think is the cause of Elizabeth’s AKI?
- Considering the Acute Dialysis Quality Initiative (ADQI) AKI staging, what stage do you consider Elizabeth to have presented with and why?
- What do you consider to be the priorities for her management?
Definitions of kidney injury
Acute kidney injury (AKI) is a clinical syndrome characterised by a rapid reduction in renal excretory function underpinned by a variety of causes. It is one of the most common secondary problems seen within critical care (Kellum et al. 2007). The term AKI replaces the long-standing term acute renal failure (ARF) (Ostermann and Chang 2007). The Acute Kidney Injury Network (AKIN) diagnostic criterion for AKI is an abrupt (within 48 hours) reduction in kidney function currently defined as an absolute increase in serum creatinine (SCr) of either ≥26.4\umumol/L or a percentage increase of ≥50% (1.5-fold from baseline) with or without oliguria (documented as <0.5 mL/kg/h for >6 hours).
The original AKI classification system known by the acronym RIFLE was proposed by the ADQI group (Bellomo et al. 2004). It was designed to be a simple and readily available tool. More recently, this classification has been updated by the AKIN group (Mehta et al. 2007) (see Table 9.2). The three new stages (1–3) map to the original first three stages of RIFLE, and define the grades of increasing severity of AKI based on changes in both SCr and urine output from a known baseline. The ‘loss’ and ‘end-stage kidney disease’ (ESKD) categories (from the original RIFLE classification) can be seen as outcomes, with ‘Loss’ being defined as a need for RRT for more than 4 weeks and ESKD where dialysis is required for longer than 3 months (Bellomo et al. 2004). Patients receiving RRT are automatically classified as being in AKIN stage 3 (Table 9.2).
Table 9.2 AKIN AKI staging
Source: Adapted from Mehta et al. (2007).
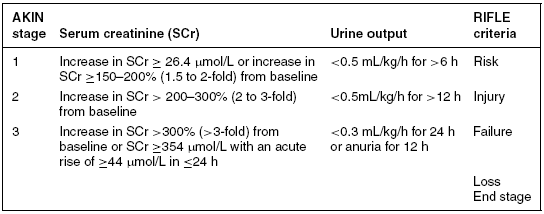
There a number of problems related to the use of this classification system. Firstly, a significant number of patients are admitted without any baseline measurement of renal function, although Ostermann and Chang (2007) argue this can be overcome by assuming a normal baseline level. Additionally, not all patients will be catheterised thus output cannot be measured. Further, the sensitivity and specificity of the classification system will be lost when diuretics are used (Ricci et al. 2008). These factors need to be taken into account during their use.
Patients are staged based on the worst value: creatinine or urine output. As her urine output is <0.5 mL/kg/h, despite not knowing her baseline creatinine, Elizabeth can be placed at stage II (see Table 9.2).
The pathophysiology of acute kidney injury
The first priority of management is to detect early signs of kidney failure (NCEPOD 2009). Elizabeth’s urine output has significantly decreased and she has passed only 100 mL in the last 6 hours (<20 mL/h). A urine output of 0.5mL/kg/h (30 mL/h) is required for Elizabeth to achieve adequate clearance of nitrogenous waste products, which, over 6 hours, would be 180 mL. Also her SCr is 125 \umumol/L, a 1.5 times increase from her normal baseline (see the section ‘Tests and investigations’ below). Elizabeth’s sodium and urea are also raised, and she has a metabolic acidaemia. This data could suggest hypovolaemia with or without kidney impairment.
The causes of AKI are classically divided into pre-kidney, intrinsic and post-kidney. The relative incidence of each of these is dependent on age, gender and clinical setting. Pre-kidney refers to a reduction in perfusion pressure. This can be due to a low cardiac output, caused by poor contractility, intravascular volume depletion or systemic vasodilation. In their multi-national study, Uchino et al. (2005) found the commonest contributing factor to ARF within the critical care environment to be hypovolaemia secondary to septic shock. Pre-kidney injury can also be caused by constriction of the afferent arteriole due to stress, drug use, for example, NSAIDs and/or the normal compensatory response initiated due to nitric oxide induced vasodilation (e.g. during sepsis) (Armitage and Thomson 2003). This leads to a kidney perfusion pressure below the auto-regulatory level required. What this level is will be determined by a number of individual factors such as age, ethnicity and pre-morbid status. However, a mean arterial blood pressure (MABP) up to 80 mmHg may be required (Bellomo et al. 2008). The most likely cause of Elizabeth’s AKI is sepsis. Her elevated temperature, white cell count and C-Reactive Protein (CRP) are evidence of infection and her poor fluid input, hypotension and tachycardia are indicative of secondary hypovolaemia. She also regularly takes non-steroidal anti-inflammatory drugs (NSAIDs) in the form of Ibuprofen, potentially affecting prostaglandin release and causing renal vasoconstriction (Huerta et al. 2005).
Intrinsic AKI refers to damage (potentially reversible) to the structures of the nephron, such as the glomeruli, tubules, vessels or interstitium; the major cause of which is acute tubular necrosis (ATN). ATN is commonly induced by ischaemic or nephrotoxic injury to the kidney and is a specific histopathological and pathophysiological entity. Nephrotoxic drugs include NSAIDs, aminoglycosides, ACE inhibitors and X-ray contrast mediums. The term ATN is considered by many authors as an inaccurate term because necrosis is rarely seen in biopsies (Armitage and Thomson 2003). Tubular cells do, however, actually die, and fall into the filtrate where they form casts that then block the lumen. Although these cells have the ability to regenerate, this can take a number of weeks. During this period kidney function is severely decreased and some form of renal support therapy may be required. Pre-kidney or ischaemic ATN often occurs as a continuum of the same pathophysiological process and together they account for the majority of AKI cases. Elizabeth’s potassium is already 5mmol/L and she has an associated metabolic acidaemia indicating a reduction in normal tubular function. If her hypovolaemia and sepsis are not resolved swiftly, then she will develop ATN.
Finally post-kidney relates to outflow obstruction in the lower urinary tract. Examples include prostatic hypertrophy and bilateral ureteric strictures. Obstructions generate a backpressure into the kidney raising the hydrostatic pressure in the Bowman’s capsule and reducing the glomerular filtration rate (GFR). Although post-kidney obstruction is an unlikely primary cause of AKI in Elizabeth, critical care nurses should be aware of the potential obstructions which could be induced by blocked indwelling urinary catheters or other unrelated conditions that Elizabeth might suffer from. For example, fibroids or other gynaecological problems can obstruct urinary flow. If there is no obvious cause, an ultrasound scan of the kidney and renal tract would be indicated to aid diagnosis.
Further details of normal kidney physiology and AKI can be found in Perkins and Kisiel (2005).
Tests and investigations
Serum creatinine (SCr)
Creatinine is produced by muscle cell breakdown, and is filtered out of the bloodstream by the kidneys. The serum level of creatinine therefore depends on a balance between the amount produced and the efficiency of filtration in the kidneys (National Kidney Federation 2008).
Daily production of creatinine depends on how much muscle the person has. Normal SCr can range between 60 and 120 \umumol/L (National Kidney Federation 2008). A 48-year-old white female such as Elizabeth would be expected to have a SCr of approximately 64 \umumol/L (Renal Association 2008). Assuming that she has a normal GFR for her age, sex and ethnic grouping, Elizabeth’s SCr, measured at 125 \umumol/L, is an increase of >1.5 times from baseline.
Glomerular filtration rate (GFR)
This is considered to be the best index to measure kidney function. It is the sum of the filtering capacity of all individual ‘healthy’ nephrons. A normal GFR is approximately 120 mL/min. GFR declines naturally with age by about 1mL/min per year. The majority of GFRs are now calculated by estimation (eGFR) as this only requires knowledge of the SCr, gender, age and ethnicity (black or other) (Renal Association 2008). Elizabeth’s eGFR can therefore be calculated as 42 mL/min per 1.73 m2 or approximately 42% of her kidney function. It should, however, be noted that eGFR may not be reliable in AKI as there is no steady state from which to calculate it.
Urea
Urea is a product of protein metabolism, usually cleared by the kidney, and therefore serum levels are usually raised in kidney injury (normal 3.5–5 mmol/L). Urea can, however, also be raised for other reasons, for example dehydration, red cell breakdown and/or liver impairment making it a less reliable indicator of kidney function (Perkins and Kisiel 2005).
Urinalysis
Urinalysis must be performed on all patients who can produce urine (NCEPOD 2009). The urine should be observed both for volume and colour. A dark colour commonly indicates increased concentration due to hypovolaemia, which can be confirmed by noting the specific gravity. Renal tubular epithelial cells and casts may also be seen in the form of ‘sediment’ during ATN as these cells have been sheared off from the inside of the tubule. Urinalysis is therefore a mandatory tool to assist in investigation of the underlying pathology. The presence of significant protein further suggests intrinsic glomerular disease, while haematuria in the presence of proteinuria may indicate a glomerular aetiology for AKI. Haematuria may also be found in lower urinary tract obstruction (post-kidney AKI). It is often associated with tumours, and also less commonly with calculi, infection or severe renal ischaemia. Red blood cells (RBC) may also be seen in cases of AKI. Myoglobinuria, a protein released from the muscle in conditions such as rhabdomyolysis, will also cause a positive reaction for blood without evidence of red cells on urine microscopy (Renal Association 2008). Any abnormalities detected on the urinalysis strip should be followed up by sending a sample to the laboratory to assess for microscopy, culture and sensitivity.
New biochemical markers of kidney injury
Waiker and Bonventre (2008) suggest the change from the term kidney failure to injury is a paradigm shift as failure refers to the kidneys’ inability to perform glomerular filtration, while injury does not. Such a shift is in line with new biochemical markers of kidney injury, which it is hoped will enable a diagnosis to be made before changes are seen in either the SCr or the urine output. SCr and urine output, although considered the best markers of kidney function at present, do not provide any information on the type or site of injury to the kidney.
New potential markers should enable an earlier diagnosis as they will be measured in the urine as a result of injury and not cellular death. The epithelial cells that line the tubules contain numerous microvilli, which contain proteins with enzymatic functions, and it is these enzymes that it is hoped can be isolated and measured to detect early damage (Waiker and Bonventre 2008). Markers that are currently being researched and that hold promise are urine biomarkers; N-acetyl-β-D-glucosaminidase (NAG), neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule – 1 (KIM-1) and serum cystatin C. It is hoped that these future markers will aid the diagnosis of the disorder before any functional decline is measured (Kellum 2008).
Developing scenario
Twenty-four hours later, Elizabeth’s condition has deteriorated further. She has received two colloid fluid challenges (each of 500 mL). She is, however, increasingly tachycardic (128 bpm), hypotensive (MABP 58 mmHg), and acidaemic (metabolic) and she has become anuric. Her Central Venous Pressure (CVP) is 12 mmHg and she has widespread crackles on auscultation, with a respiratory rate of 20 breaths per minute. Laboratory results reveal the following:
| Creatinine: | 300 \umumol/L |
| Urea: | 25 mmol/L |
| Potassium: | 5.8 mmol/L |
| Sodium: | 147 mmol/L |
| Hb: | 10.5 g/dL |
A decision is made to transfer her to the ICU for further management.
Managing the patient with AKI
Airway/breathing
Elizabeth will require administration of supplemental oxygen titrated against regular arterial blood gases to help ensure adequate pulmonary gas exchange and maintenance of renal cell oxygenation (Perkins and Kisiel 2005). The crackles heard on auscultation of Elizabeth’s lung fields are likely to indicate pulmonary oedema. If this is significantly affecting her oxygenation then a loop diuretic such as frusemide might be prescribed to offload the fluid and restore gas exchange. The nurse should also be alert for signs of a chest infection, which could be indicated by the increase in respiratory rate and widespread crackles, particularly as Elizabeth was admitted with community-acquired pneumonia. Assistance with positioning, coughing and suctioning, in conjunction with physiotherapy input, should therefore be provided as required. Elizabeth may also require non-invasive or invasive ventilation to maintain adequate oxygenation and ventilation if the fluid overload combined with the pneumonia and an attempt to breathe off the metabolic acid (Kussmual respirations), excessively increases her work of breathing, and worsens her blood gases.
Circulation
Fluid resuscitation
Optimal intravascular volume is vital to ensure adequate renal perfusion. Fluid resuscitation should be initiated as soon as hypoperfusion is recognised. The majority of cases of AKI can be effectively treated and resolved by adequate volume replacement and treatment of the underlying medical condition. Vincent and Weil (2006) suggest there are four phases of decision making in relation to fluid administration: type of fluid, either crystalloid or colloids; rate of infusion; goal to be achieved, for example MABP and CVP targets and safety limits. The type of fluid required is, according to Bellomo et al. (2004), controversial. Bagshaw and Bellomo (2007), for example, argue that synthetic colloids such as hydroxyethyl starch (HES) may increase the risk of AKI. They also state that the timing and amount of fluid therapy may also affect outcome (Bagshaw and Bellomo 2007). Fluid replacement may best be achieved through the rapid infusion of repeated small volumes (250 mL of crystalloid or colloid) of fluid alongside close monitoring of CVP, urinary output and other end points. Fluid challenges should be administered and repeated based on response (Vincent and Gerlach 2004), and administration of fluid should continue until identified targets have been reached (Vincent and Weil 2006).
Fluid challenges and resuscitation targets (early goal-directed therapy)
A fluid challenge is quite distinct from fluid administration, the purpose being to ascertain the intravascular fluid status of a patient. Dellinger et al. (2008) describe it as a technique in which large amounts of fluids are administered over a defined time period with the patients’ response to the challenge being closely observed. For a fluid challenge, Dellinger et al. (2008) recommend 20 mL/kg of crystalloid over 5–10 minutes titrated to haemodynamic goals.
Nurses should have an understanding of the optimal CVP target and other fluid resuscitation goals. In 1979, Weil and Henning argued that it was necessary to achieve a sustained CVP rise of at least 2 mmHg to confirm adequate circulating volume (Weil and Henning 1979). Much has been written since regarding the improvement in survival by patients with the introduction of early goal-directed therapy, whereby end goals are set to determine success of the determined intervention (e.g. Rivers et al. 2001). Recently, the surviving sepsis guidelines have advocated a CVP target of 8–12 mmHg for septic patients (Dellinger et al. 2008). Other fluid resuscitation goals include lactate < 4 mmol, MABP >65 mmHg, central venous oxygen saturations >70% and a urine output >0.5mL/kg/min (Dellinger et al. 2008). In some critical care areas, more sophisticated tools might also be available to aid fluid assessment. These include, for example, measurement of pulmonary artery occlusion pressure (PAOP) using a pulmonary artery catheter; extra-vascular lung water (EVLW) using the PiCCO system (Pulsion medical) and stroke volume variation (SVV) and/or pulse pressure variation (PPV) using the LiDCO system. The value of non-invasive assessment should also be remembered, and clinical examination should therefore include measurement of jugular venous pressure (JVP), capillary refill time (CRT) and assessment of peripheral warmth and perfusion wherever possible.
Although Elizabeth has received 1 L of colloid, there has been no improvement in her kidney function. She also has a worsening metabolic acidaemia, which is likely to be of a mixed cause (both lactic and renal). This could suggest that more fluid may be required to improve oxygen delivery, especially as she has signs of sepsis, which requires administration of large volumes of fluid (Dellinger et al. 2008). She already has a CVP of 12 mmHg and signs of pulmonary oedema, however, suggesting that other treatments also now need consideration.
Optimal management of both fluids and electrolytes can be challenging during AKI, and will require different strategies during each phase of the illness (anuric/oliguric, diuretic, recovery). During the anuric/oliguric phase, once adequate intravascular filling has been established, Elizabeth may require a strategy of fluid restriction to prevent fluid volume overload (Hall and Esser 2008). It is first important, however, to ensure that the anuria is not caused by a blocked indwelling urinary catheter. If fluid volume overload is suspected, then frusemide may be prescribed to reduce clinical symptoms (Small and McMullen 2005). Frusemide is a loop diuretic which works to maintain renal blood flow and urine output, preventing further tubule obstruction. However, inappropriate administration risks a further reduction in renal perfusion worsening the AKI (Small and McMullen 2005). It should therefore be used with caution, and only when a thorough assessment to ensure adequate intravascular volume has been established. Dopamine has also been used for this purpose, but current research evidence does not support its continued use (Kellum and Decker 2001). If Elizabeth enters the diuretic phase, additional fluids may again be necessary to prevent dehydration. As the urine may still be of poor quality, electrolyte dysfunction can continue to be problematic during this period. Strict attention to monitoring and recording all input, output and electrolyte values will be vital throughout all phases.
Red blood cells (RBC) might be prescribed for Elizabeth if she becomes anaemic. Anaemia can be due to decreased erythropoietin production and a shortened RBC life span (Ward 2005). In AKI, however, it is more likely to be due to frequent blood sampling and/or haemodilution. In septic patients, the usually accepted strategy is to aim for an Hb >7 g/dL (Hébert et al. 1999). Red cell administration will assist in optimising her intravascular filling, but may also contribute to further total body fluid overload. Multiple units could also exacerbate the risk of infection, and could worsen the already evident hyperkalaemia. Hyperkalaemia is of concern as it may cause cardiac dysrhythmias, and thus requires vigilant cardiac monitoring and potential pharmacological treatment. It can be treated by correcting the metabolic acidaemia, and/or by the use of hypertonic glucose and insulin or nebulised salbutamol to force potassium back into the cells. Intravenous (IV) calcium might also be used to decrease the effect of potassium on the cardiac membrane (Hall and Esser 2008). Alternatively, a rectal or oral calcium resonium preparation might be prescribed which allows the exchange of sodium ions for potassium ions, thus excreting potassium into the stool (Hall and Esser 2008). Renal replacement therapy (RRT) might also be considered if the hyperkalaemia becomes life threatening.
Elizabeth’s nutritional status needs consideration as patients with AKI tend to have high energy and protein requirements, which are further increased during mechanical ventilation and sepsis (Hall and Esser 2008). Perkins and Kisiel (2005) note, however, that a diet should be offered that has sufficient calorific content to meet the increased metabolic demand (higher in fat and carbohydrates), whilst being low in phosphate and protein to prevent further protein metabolism, and low in sodium to prevent further water retention. The type of nutritional input will also depend on whether or not Elizabeth is receiving RRT. Enteral feeding should be continued where possible as this will assist in preventing secondary sepsis from occurring and reduce the risk of gastrointestinal bleeding. Optimal nutritional management will require dietetic advice. Specialist enteral nutrition formulas such as Nepro® are available for use, but should be stopped during RRT. They are also expensive and should not be used without consideration to their relative benefits. Concentrated feed formulas can also help in the oliguric patient who is not receiving RRT. Detailed guidance for use of enteral nutrition in AKI is available from the European Society for Clinical Nutrition and Metabolism (ESPEN) (Cano et al. 2006).
Disability of the central nervous system
A close watch on the neurological status of Elizabeth is vital during her treatment for AKI. Rising urea and haemodynamic instability alongside an increased tendency for anaemia and bleeding and the potential for toxicity may cause symptoms of drowsiness, coma, psychosis and possible seizure activity (Hall and Esser 2008). Accurate neurological assessment may be more challenging, however, if Elizabeth requires intubation with associated sedation. Blood glucose levels should also be monitored regularly as insulin resistance can develop, leading to hyperglycaemia (Hall and Esser 2008).
Exposure/environment
Whilst in critical care, the risk of Elizabeth contracting an infection will be high, not least due to the large number of invasive cannulae she is likely to have. She may also be immunocompromised due to uraemia (Perkins and Kisiel 2005). Infection is an important contributor to poor mortality in this patient group (Hall and Esser 2008). Unnecessary lines should be removed whenever possible to reduce this risk, and universal precautions and guidelines for maintaining asepsis should be adhered to at all times (see Chapter 4 for more information on sepsis). If Elizabeth is anuric, removal of the urinary catheter during RRT should be considered. Assistance with personal hygiene and good skin care is vital for Elizabeth, particularly as she may have itchy, dry flaky skin coupled with areas of dependent oedema (Redmond et al. 2004).
Owing to the increasing inability of her kidneys to excrete metabolic waste products, a review of all Elizabeth’s medication, in particular the use of antibiotics and NSAIDs, should be undertaken in order to identify any potential alterations to drugs or doses that might be required (Perkins and Kisiel 2005). Involvement of the pharmacist will be vital, both during RRT when normal drug doses may be possible and when not receiving RRT, as doses may then need to be reduced to prevent higher than acceptable serum drug levels.
Renal replacement therapy (RRT)
The best way to control Elizabeth’s worsening metabolic acidosis, increasing urea abnormal electrolytes, in particular the hyperkalaemia, and fluid overload is to commence RRT. The AKIN classification does not indicate when RRT should be initiated. In their retrospective review of >41 000 patients, however, Ostermann and Chang (2007) noted that the mean GFR at the time of initiation of RRT was 23.8mL/min/1.73m2. There are, however, large variations in practice between units and within departments (Ostermann and Chang 2007). In their guidelines on septic shock, Dellinger et al. (2008) suggest that continuous renal replacement therapies (CRRTs) can be used over intermittent haemodialysis in patients with severe sepsis and AKI, despite the lack of randomised studies. The majority of ICUs (78%) in the United Kingdom use continuous veno-venous haemofiltration (CVVH) for this purpose (Gatward et al. 2008).
What is CVVH?
Helen Dickie, a critical care renal nurse specialist at Guys and St Thomas NHS Foundation Trust in London, describes CVVH in the following way:
The patient is attached to an extracorporeal blood circuit in which blood is pumped through a haemofilter (see Figures 9.1 and Figures 9.2, for examples, of haemofiltration machines). It is a veno-venous circuit, connected either to two separate central venous catheters or, more commonly, to one dual lumen central venous catheter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree