Chapter 25 On completion of this chapter, the reader will be able to: • Summarize assessment and care of the newborn with soft tissue, skeletal, and neurologic injuries caused by birth trauma. • Identify maternal conditions that place the newborn at risk for infection. • Describe methods used to identify infection in the newborn. • Identify the effects of maternal use of alcohol, heroin, methadone, marijuana, methamphetamine, cocaine, and tobacco on the fetus and newborn. • Describe the assessment of a newborn exposed to harmful drugs in utero. • Identify clinical manifestations of infection in the newborn. • Describe the nurse’s role in the diagnosis of neonatal sepsis. • Compare characteristics of neonatal Rh and ABO incompatibility. • Compare and contrast the physical characteristics of preterm, late preterm, term, and postterm neonates. • Discuss respiratory distress syndrome and the approach to treatment. • Compare methods of oxygen therapy for the high risk infant. • Describe nursing interventions for nutritional care of the preterm infant. • Discuss the pathophysiology of retinopathy of prematurity and bronchopulmonary dysplasia and identify the predisposing risk factors. • Describe risk factors associated with the birth and transition of an infant of a diabetic mother. • Plan developmentally appropriate care for the high risk infant. • Develop a plan to address the unique needs of parents of high risk infants. • Describe nursing care of the family in the event of a stillbirth or death of a high risk infant. • Discuss the identification and care of infants with an inborn error of metabolism. High risk infants are most often classified according to birth weight, gestational age, and predominant pathophysiologic problems. The more common problems related to physiologic status are closely associated with the state of maturity of the infant and usually involve chemical disturbances (e.g., hypoglycemia, hypocalcemia) or consequences of immature organs and systems (e.g., hyperbilirubinemia, respiratory distress, hypothermia). Because high risk factors are common to several specialty areas—particularly obstetrics, pediatrics, and neonatology—specific terminology is needed to describe the developmental status of the newborn (Box 25-1). Many injuries are minor and resolve readily in the neonatal period without treatment. Other trauma requires some degree of intervention; few are serious enough to be fatal. The nurse’s contributions to the newborn’s welfare begin with early observation of his or her transition. Promptly reporting signs that indicate deviations from normal permits early initiation of appropriate therapy. Table 25-1 provides an overview of neurologic birth injuries and the sites in which they occur. TABLE 25-1 From Paige PL, Moe PC: Neurologic disorders. In Merenstein GB, Gardner SL, editors: Handbook of neonatal intensive care, ed 6, St Louis, 2006, Mosby. Soft-tissue injuries that commonly occur at birth (i.e., caput succedaneum and cephalhematoma) are discussed in Chapter 22. The clavicle is the bone most often fractured during birth. Generally the break is in the middle third of the bone (Fig. 25-1). Dystocia, particularly shoulder impaction, may be the predisposing problem. Limited arm motion, crepitus over the bone, and absence of the Moro reflex on the affected side are often present. Except for use of gentle rather than vigorous handling and containment of the limb against the chest, no accepted treatment for fractured clavicle of the newborn exists, and the prognosis is good. The humerus and femur are other bones that may be fractured during a difficult birth. Fractures in newborns generally heal rapidly. Immobilization is accomplished with slings, splints, swaddling, and other devices. The clinical manifestations of Erb palsy are related to the paralysis of the affected extremity and muscles. The arm hangs limp alongside the body. The shoulder and arm are adducted and rotated internally. The elbow is extended, and the forearm is pronated with the wrist and fingers flexed; a grasp reflex may be present because finger and wrist movement remains normal (Carlo, 2011b) (Fig. 25-2). In lower-plexus palsy the muscles of the hand are paralyzed, with consequent wrist drop and relaxed fingers. In a third and more severe form of brachial palsy, the entire arm is paralyzed and hangs limp and motionless at the side. The Moro reflex is absent on the affected side for all of the forms of brachial palsy. Total plexus is the second most common type of plexus injury. Treatment of the affected arm is aimed at preventing contractures of the paralyzed muscles and maintaining correct placement of the humeral head within the glenoid fossa of the scapula. Complete recovery from stretched nerves usually takes 3 to 6 months. However, avulsion of the nerves (complete disconnection of the ganglia from the spinal cord that involves both anterior and posterior roots) results in permanent damage. For injuries that do not improve by 3 months, surgical intervention may be needed to relieve pressure on the nerves or repair the nerves with grafting (Carlo, 2011b). Pressure on the facial nerve (cranial nerve VII) during birth may result in injury to it. The primary clinical manifestations are loss of movement on the affected side such as an inability to completely close the eye, drooping of the corner of the mouth, and absence of wrinkling of the forehead and nasolabial fold (Fig. 25-3). Facial palsy or paralysis is most noticeable when the infant cries. The mouth is drawn to the unaffected side, the wrinkles are deeper on the normal side, and the eye on the involved side remains open. Often the condition is temporary, resolving within hours or days of birth. Permanent paralysis is rare unless the nerve fibers were torn, in which case surgical intervention may be necessary. Phrenic nerve paralysis results in diaphragmatic paralysis as demonstrated by ultrasonography, which shows paradoxical chest movement and an elevated diaphragm. Initially radiography may not demonstrate an elevated diaphragm if the neonate is receiving positive-pressure ventilation (Volpe, 2008). The injury sometimes occurs in conjunction with brachial palsy. Respiratory distress is the most common and important sign of injury. Because injury to the phrenic nerve is usually unilateral, the lung on the affected side does not expand, and respiratory efforts are ineffectual. The infant is positioned on the affected side to facilitate maximum expansion of the uninvolved lung. Breathing is primarily thoracic; cyanosis, tachypnea, or complete respiratory failure may be seen. Pneumonia and atelectasis on the affected side may also occur. The infant with phrenic nerve paralysis requires the same nursing care as any infant with respiratory distress. As with other birth injuries, the family’s emotional needs are similar to those discussed for soft-tissue injury (see Chapter 22). Follow-up is also essential because of the extended length of recovery. Neurologic injury in newborn infants is common with the increased survival of low-birth-weight and very low–birth-weight infants; in addition, the lower the gestational age, the higher the risk for certain neurologic injuries. Such infants are particularly vulnerable to ischemic injury caused by variable (both increased and decreased) cerebral blood flow subsequent to asphyxia; and preterm infants, with a fragile cerebrovascular network, are highly prone to periventricular or intraventricular hemorrhage. Fragility and increased permeability of capillaries and prolonged prothrombin time predispose preterm infants to trauma when delicate structures are subjected to the forces of labor. The more common cerebral complications and nursing care are outlined in Table 25-2. TABLE 25-2 The highest incidence of abnormal neurologic findings occurs in very low–birth-weight (VLBW) infants and those with intracranial hemorrhage. Major neurologic problems such as cerebral palsy, seizures, and hydrocephalus are usually diagnosed in the first 2 years of life. Less severe deficits such as learning disorders, attention deficit hyperactivity disorder (ADHD), and fine- and gross-motor incoordination may not be diagnosed until preschool or even school age. Cerebral palsy is one of the most common neurologic deficits in survivors of preterm birth (see Chapter 49). Recent research has shown that therapeutic hypothermia provided by cooling either the infant’s head or the whole body reduces the severity of the neurologic damage in hypoxic ischemic encephalopathy when it is applied in the early stages of injury (first 6 hours after birth) in infants with a gestational age of 35 to 36 weeks or more (Azzopardi, Strohm, Edwards, et al., 2009; Edwards, Brocklehurst, Gunn, et al., 2010; Jacobs, Hunt, Tarnow-Mordi, et al., 2007; Laptook, 2009). Table 25-3 outlines risk factors for neonatal sepsis. Special precautions for preventing infection and prompt recognition when it occurs are necessary for optimal newborn care. Neonatal infections may be acquired in utero, at birth or shortly thereafter, and as a health care–associated infection (HAI). Neonatal bacterial infection is classified into two patterns according to the time of presentation. Early-onset or congenital sepsis usually manifests within 24 to 48 hours of birth, progresses more rapidly than later-onset infection, and carries a mortality rate as high as 50%. Early-onset sepsis is acquired in the perinatal period; infection can occur from direct contact with organisms from the maternal GI and genitourinary tracts. The most common infecting organisms are Escherichia coli and group B streptococcus (GBS) (Stoll, Hansen, Sanchez, et al., 2011). E. coli, which may be present in the vagina, accounts for approximately half of all cases of sepsis caused by gram-negative organisms. GBS is an extremely virulent organism in neonates, with a high (50%) death rate in affected infants. Other bacteria noted to cause early-onset infection include Haemophilus influenzae, Citrobacter and Enterobacter organisms, coagulase-negative staphylococci, and Streptococcus viridans. Other pathogens that are harbored in the vagina and may infect the infant include gonococci, Candida albicans, herpes simplex virus (HSV) type 2, and chlamydia. Early-onset sepsis is associated with a history of obstetric events such as preterm birth, prolonged rupture of membranes (more than 18 hours), maternal fever during labor, and chorioamnionitis. Early-onset infection is also inversely related to infant birth weight (Polin and AAP Committee on Fetus and Newborn, 2012). Late-onset sepsis, occurring approximately at 7 to 30 days of age, is considered primarily to be an infection acquired in the hospital or community; the offending organisms are usually staphylococci, Klebsiella organisms, enterococci, E. coli, and Pseudomonas or Candida species (Stoll, 2011). Coagulase-negative staphylococci, considered to be primarily a contaminant in older children and adults, are commonly found to be the cause of septicemia in extremely low–birth-weight (ELBW) and VLBW infants. Additional infections of concern include methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, and multidrug-resistant gram-negative pathogens. Bacterial invasion can occur through sites such as the umbilical stump; the skin; mucous membranes of the eye, nose, pharynx, and ear; and internal systems such as the respiratory, nervous, urinary, and GI systems. The earliest clinical signs of neonatal sepsis are characterized by a lack of specificity. The nonspecific signs include lethargy, poor feeding, poor weight gain, and irritability. The nurse or parent may simply note that the infant is not doing as well as before. Differential diagnosis may be difficult because signs of sepsis are similar to signs of noninfectious neonatal problems such as hypoglycemia and respiratory distress. Additional clinical and laboratory information, including cultures, supplement the findings described. Table 25-4 outlines the clinical manifestations associated with neonatal sepsis. TABLE 25-4 CLINICAL MANIFESTATIONS OF SEPSIS* *Laboratory findings include neutropenia, increased bands, hypoglycemia or hyperglycemia, metabolic acidosis, and thrombocytopenia. Modified from Askin DF: Bacterial and fungal sepsis in the neonate, J Obstet Gynecol Neonatal Nurs 24(7):635–643, 1995. Vigilant assessment continues during and after treatment. Prolonged administration of antibiotics to ELBW neonates without positive cultures in the first week of life is associated with an increased incidence of necrotizing enterocolitis (NEC), mortality, and late-onset infection; therefore careful use of antibiotics and close observation of such infants are recommended (Cotten, Taylor, Stoll, et al., 2009; Kuppala, Meinzen-Derr, Morrow, et al., 2011). The newborn continues to be assessed for sequelae to septicemia, which include meningitis, disseminated intravascular coagulation (DIC), NEC, pneumonia, and septic shock. Septic shock results from the toxins released into the bloodstream. The most common signs include decreasing oxygen saturation, poor perfusion (prolonged capillary refill, cool extremities, mottling), tachycardia, respiratory distress, and hypotension. Breastfeeding (medically stable infants) or feeding the newborn expressed breast milk from the mother is encouraged. Breast milk provides protective mechanisms. Colostrum contains IgA, which offers protection against infection in the GI tract. Human milk contains iron-binding protein that exerts a bacteriostatic effect on E. coli. Human milk also contains macrophages and lymphocytes. The vulnerability of infants to common mucosal pathogens such as RSV may be reduced by passive transfer of maternal immunity in the colostrum and breast milk. There is evidence that early enteral feedings with human milk are beneficial in establishing a natural barrier to infection in ELBW and VLBW infants (Hanson, 2007). Human milk is also thought to provide some degree of protection from NEC. Care must be taken in suctioning secretions from any newborn’s oropharynx or trachea. Routine suctioning is not recommended and may further compromise the infant’s immune status, cause hypoxia, and increase ICP. Efforts should also be taken to prevent ventilator-associated pneumonia in infants on mechanical ventilation (see Chapter 40). Isolation procedures are implemented as indicated according to hospital policy. Isolation protocols change rapidly, and the nurse is urged to participate in continuing education and in-service programs to remain up to date. Virtually all controlled clinical trials have demonstrated that effective hand washing is responsible for the prevention of HAI in nursery units. Nursing is directly or indirectly responsible for minimizing or eliminating environmental sources of infectious agents in the nursery. Measures to be taken include Standard Precautions, careful and thorough cleaning of contaminated equipment, frequent replacement of used equipment (e.g., changing IV and nasogastric [NG] tubing per hospital protocol and cleaning resuscitation and ventilation equipment, IV pumps, and incubators), and appropriate disposal of contaminated linens and diapers. Overcrowding must be avoided in nurseries. Guidelines for space, visitation, and general infection control in areas where newborns receive care have been established and published (AAP and ACOG, 2007). Infants cared for in the NICUs are at high risk for infection. Infection rates in VLBW infants are higher in those with lower birth weights. Hand washing is the single most effective measure to reduce HAI. However, the rate of compliance with standards for hand hygiene is reported to be only 22% (Buus-Frank, 2004). The combined use of alcohol, hand hygiene, and gloves is effective in reducing the incidence of systemic infection. It is incumbent on caregivers to strictly adhere to recommended guidelines for hand hygiene. Antibiotic is instilled into a newborn’s eyes 1 to 2 hours after birth to prevent infection (see Fig. 23-5). The skin, its secretions, and normal flora are natural defenses that protect against invading pathogens. Warm water may be used to remove blood and meconium from the neonate’s face, head, and body. A mild nonmedicated soap (in a single-use container) can be used with careful water rinsing. Vernix caseosa is not scrubbed vigorously for removal, since this further disrupts the skin barrier properties (see Guidelines box, pp. 701-702). No single method of cord care has been shown to be more effective in the promotion of drying, separating, and preventing colonization. Current recommendations for cord care by the Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN) (2007) include cleaning the cord with sterile water or a neutral pH cleanser; subsequent care entails cleaning the cord with water. Nurses must follow agency protocols for cord care, but they can recommend revision of protocols based on research (see also Umbilical Cord Care, Chapter 23, p. 627). Polin, Denson, Brady, et al. (2012) provide additional strategies for the prevention of HAI in the NICU. To determine the causative agent in a symptomatic infant, tests are performed to rule out each of these infections. The O category may involve testing for several viral infections (e.g., HBV, varicella zoster, measles, mumps, HIV, syphilis, and human parvovirus). Bacterial infections are not included in the TORCH workup because they are usually identified by clinical manifestations and readily available laboratory tests. Gonococcal conjunctivitis (ophthalmia neonatorum) and chlamydial conjunctivitis have been reduced significantly by prophylactic measures at birth (see Critical Thinking Case Study and Chapter 22). The major maternal infections, their possible effects, and specific nursing considerations are outlined in Table 25-5. TABLE 25-5 INFECTIONS ACQUIRED FROM THE MOTHER BEFORE, DURING, OR AFTER BIRTH* *This table is not an exhaustive representation of all perinatally transmitted infections. For further information regarding specific diseases or treatment not listed here, refer to American Academy of Pediatrics (AAP) Committee on Infectious Diseases, Pickering L, editor: 2012 Red book: report of the Committee on Infectious Diseases, ed 29, Elk Grove Village, Ill, 2012, AAP.
The High Risk Newborn
Birth Injuries
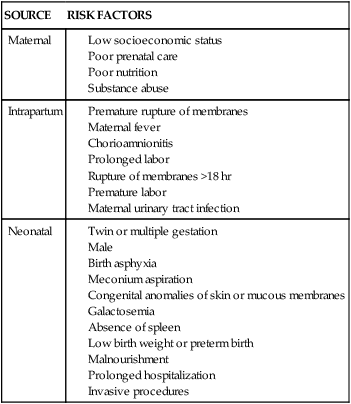
SITE OF INJURY
TYPE OF INJURY
Scalp
Skull
Intracranial
Spinal cord (cervical)
Plexus
Cranial and peripheral nerve
Care Management
Skeletal Injuries
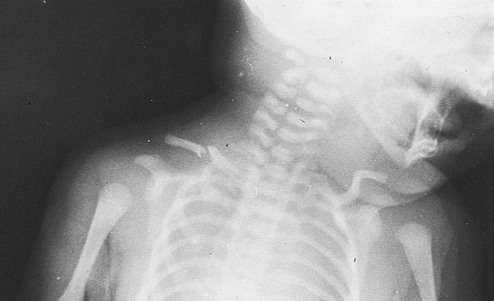
Peripheral Nervous System Injuries
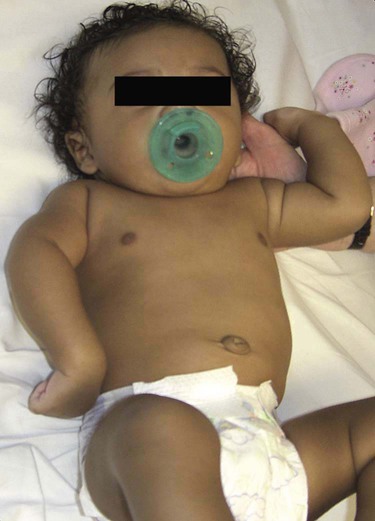
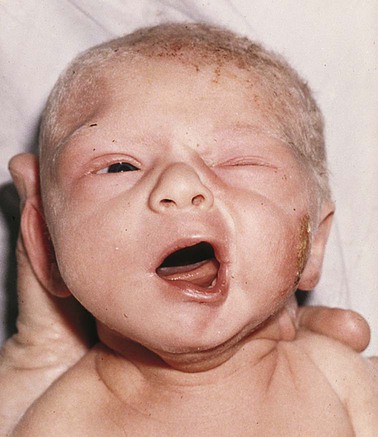
Neurologic Injuries
DESCRIPTION
CLINICAL MANIFESTATIONS
THERAPEUTIC MANAGEMENT
CARE MANAGEMENT
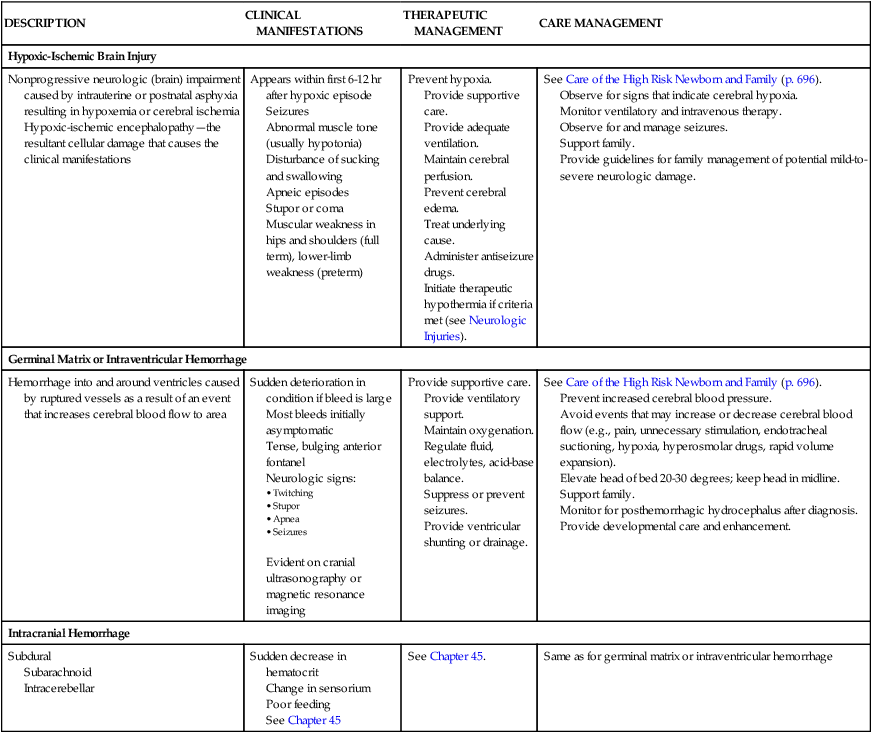
Hypoxic-Ischemic Brain Injury
Nonprogressive neurologic (brain) impairment caused by intrauterine or postnatal asphyxia resulting in hypoxemia or cerebral ischemia
Hypoxic-ischemic encephalopathy—the resultant cellular damage that causes the clinical manifestations
Appears within first 6-12 hr after hypoxic episode
Seizures
Abnormal muscle tone (usually hypotonia)
Disturbance of sucking and swallowing
Apneic episodes
Stupor or coma
Muscular weakness in hips and shoulders (full term), lower-limb weakness (preterm)
Prevent hypoxia.
Provide supportive care.
Provide adequate ventilation.
Maintain cerebral perfusion.
Prevent cerebral edema.
Treat underlying cause.
Administer antiseizure drugs.
Initiate therapeutic hypothermia if criteria met (see Neurologic Injuries).
See Care of the High Risk Newborn and Family (p. 696).
Observe for signs that indicate cerebral hypoxia.
Monitor ventilatory and intravenous therapy.
Observe for and manage seizures.
Support family.
Provide guidelines for family management of potential mild-to-severe neurologic damage.
Germinal Matrix or Intraventricular Hemorrhage
Hemorrhage into and around ventricles caused by ruptured vessels as a result of an event that increases cerebral blood flow to area
Sudden deterioration in condition if bleed is large
Most bleeds initially asymptomatic
Tense, bulging anterior fontanel
Neurologic signs:
Evident on cranial ultrasonography or magnetic resonance imaging
Provide supportive care.
Provide ventilatory support.
Maintain oxygenation.
Regulate fluid, electrolytes, acid-base balance.
Suppress or prevent seizures.
Provide ventricular shunting or drainage.
See Care of the High Risk Newborn and Family (p. 696).
Prevent increased cerebral blood pressure.
Avoid events that may increase or decrease cerebral blood flow (e.g., pain, unnecessary stimulation, endotracheal suctioning, hypoxia, hyperosmolar drugs, rapid volume expansion).
Elevate head of bed 20-30 degrees; keep head in midline.
Support family.
Monitor for posthemorrhagic hydrocephalus after diagnosis.
Provide developmental care and enhancement.
Intracranial Hemorrhage
Subdural
Subarachnoid
Intracerebellar
Sudden decrease in hematocrit
Change in sensorium
Poor feeding
See Chapter 45
See Chapter 45.
Same as for germinal matrix or intraventricular hemorrhage
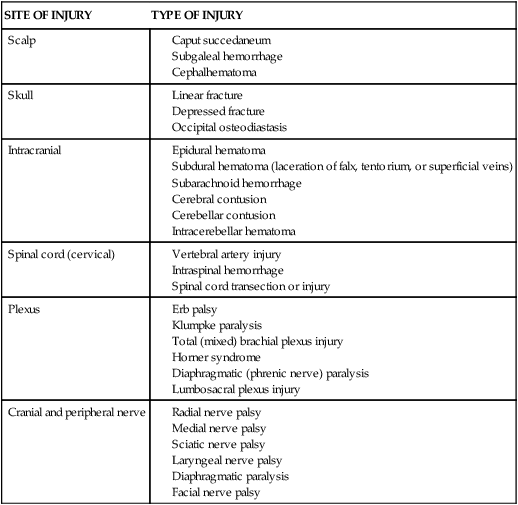
Neonatal Infections
Sepsis
Care Management
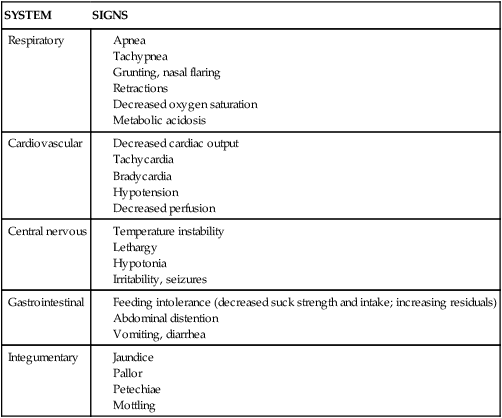
SYSTEM
SIGNS
Respiratory
Cardiovascular
Central nervous
Gastrointestinal
Integumentary
Prevention
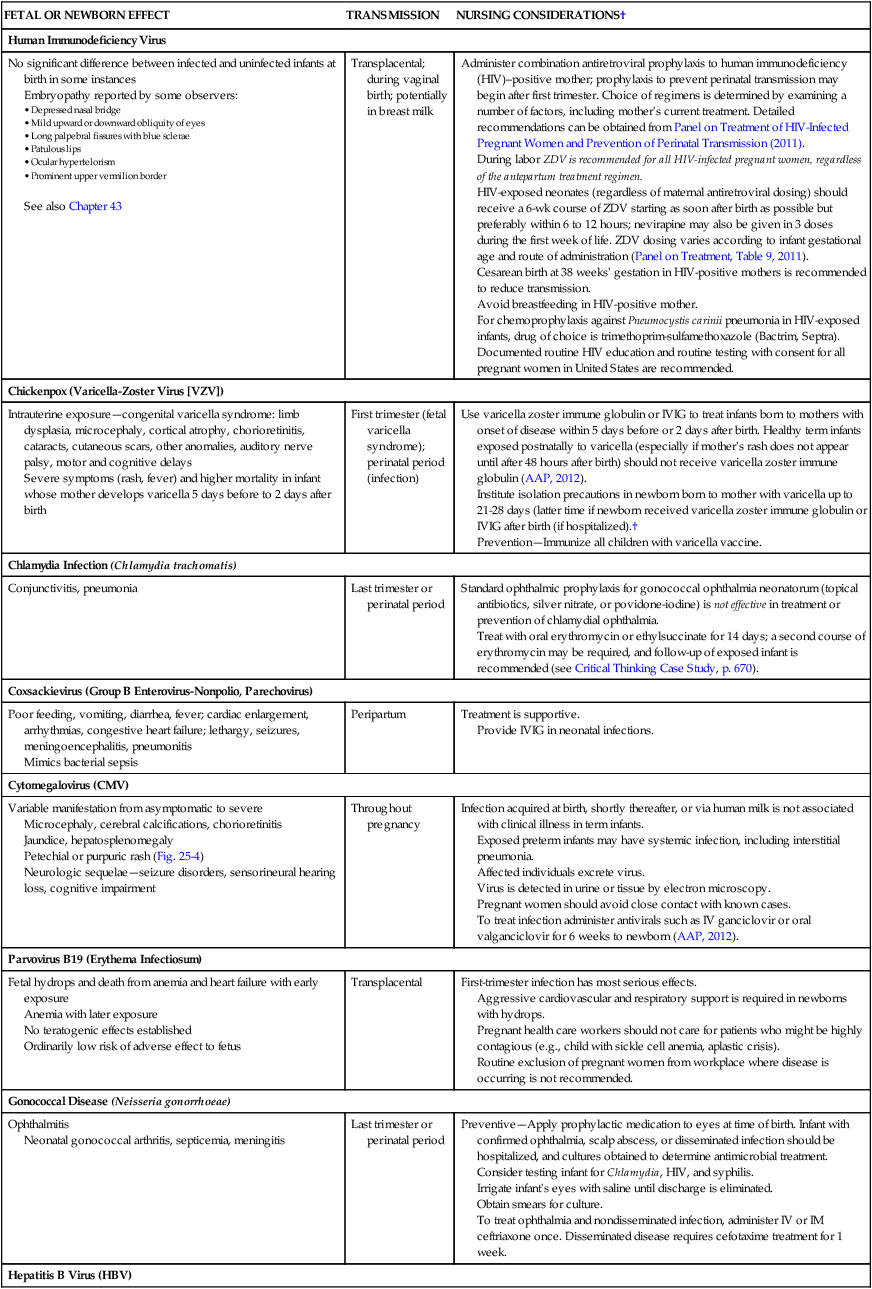
Maternal Infections
FETAL OR NEWBORN EFFECT
TRANSMISSION
NURSING CONSIDERATIONS†
Human Immunodeficiency Virus
No significant difference between infected and uninfected infants at birth in some instances
Embryopathy reported by some observers:
See also Chapter 43
Transplacental; during vaginal birth; potentially in breast milk
Administer combination antiretroviral prophylaxis to human immunodeficiency (HIV)–positive mother; prophylaxis to prevent perinatal transmission may begin after first trimester. Choice of regimens is determined by examining a number of factors, including mother’s current treatment. Detailed recommendations can be obtained from Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission (2011).
During labor ZDV is recommended for all HIV-infected pregnant women, regardless of the antepartum treatment regimen.
HIV-exposed neonates (regardless of maternal antiretroviral dosing) should receive a 6-wk course of ZDV starting as soon after birth as possible but preferably within 6 to 12 hours; nevirapine may also be given in 3 doses during the first week of life. ZDV dosing varies according to infant gestational age and route of administration (Panel on Treatment, Table 9, 2011).
Cesarean birth at 38 weeks’ gestation in HIV-positive mothers is recommended to reduce transmission.
Avoid breastfeeding in HIV-positive mother.
For chemoprophylaxis against Pneumocystis carinii pneumonia in HIV-exposed infants, drug of choice is trimethoprim-sulfamethoxazole (Bactrim, Septra).
Documented routine HIV education and routine testing with consent for all pregnant women in United States are recommended.
Chickenpox (Varicella-Zoster Virus [VZV])
Intrauterine exposure—congenital varicella syndrome: limb dysplasia, microcephaly, cortical atrophy, chorioretinitis, cataracts, cutaneous scars, other anomalies, auditory nerve palsy, motor and cognitive delays
Severe symptoms (rash, fever) and higher mortality in infant whose mother develops varicella 5 days before to 2 days after birth
First trimester (fetal varicella syndrome); perinatal period (infection)
Use varicella zoster immune globulin or IVIG to treat infants born to mothers with onset of disease within 5 days before or 2 days after birth. Healthy term infants exposed postnatally to varicella (especially if mother’s rash does not appear until after 48 hours after birth) should not receive varicella zoster immune globulin (AAP, 2012).
Institute isolation precautions in newborn born to mother with varicella up to 21-28 days (latter time if newborn received varicella zoster immune globulin or IVIG after birth (if hospitalized).†
Prevention—Immunize all children with varicella vaccine.
Chlamydia Infection (Chlamydia trachomatis)
Conjunctivitis, pneumonia
Last trimester or perinatal period
Standard ophthalmic prophylaxis for gonococcal ophthalmia neonatorum (topical antibiotics, silver nitrate, or povidone-iodine) is not effective in treatment or prevention of chlamydial ophthalmia.
Treat with oral erythromycin or ethylsuccinate for 14 days; a second course of erythromycin may be required, and follow-up of exposed infant is recommended (see Critical Thinking Case Study, p. 670).
Coxsackievirus (Group B Enterovirus-Nonpolio, Parechovirus)
Poor feeding, vomiting, diarrhea, fever; cardiac enlargement, arrhythmias, congestive heart failure; lethargy, seizures, meningoencephalitis, pneumonitis
Mimics bacterial sepsis
Peripartum
Treatment is supportive.
Provide IVIG in neonatal infections.
Cytomegalovirus (CMV)
Variable manifestation from asymptomatic to severe
Microcephaly, cerebral calcifications, chorioretinitis
Jaundice, hepatosplenomegaly
Petechial or purpuric rash (Fig. 25-4)
Neurologic sequelae—seizure disorders, sensorineural hearing loss, cognitive impairment
Throughout pregnancy
Infection acquired at birth, shortly thereafter, or via human milk is not associated with clinical illness in term infants.
Exposed preterm infants may have systemic infection, including interstitial pneumonia.
Affected individuals excrete virus.
Virus is detected in urine or tissue by electron microscopy.
Pregnant women should avoid close contact with known cases.
To treat infection administer antivirals such as IV ganciclovir or oral valganciclovir for 6 weeks to newborn (AAP, 2012).
Parvovirus B19 (Erythema Infectiosum)
Fetal hydrops and death from anemia and heart failure with early exposure
Anemia with later exposure
No teratogenic effects established
Ordinarily low risk of adverse effect to fetus
Transplacental
First-trimester infection has most serious effects.
Aggressive cardiovascular and respiratory support is required in newborns with hydrops.
Pregnant health care workers should not care for patients who might be highly contagious (e.g., child with sickle cell anemia, aplastic crisis).
Routine exclusion of pregnant women from workplace where disease is occurring is not recommended.
Gonococcal Disease (Neisseria gonorrhoeae)
Ophthalmitis
Neonatal gonococcal arthritis, septicemia, meningitis
Last trimester or perinatal period
Preventive—Apply prophylactic medication to eyes at time of birth. Infant with confirmed ophthalmia, scalp abscess, or disseminated infection should be hospitalized, and cultures obtained to determine antimicrobial treatment.
Consider testing infant for Chlamydia, HIV, and syphilis.
Irrigate infant’s eyes with saline until discharge is eliminated.
Obtain smears for culture.
To treat ophthalmia and nondisseminated infection, administer IV or IM ceftriaxone once. Disseminated disease requires cefotaxime treatment for 1 week.
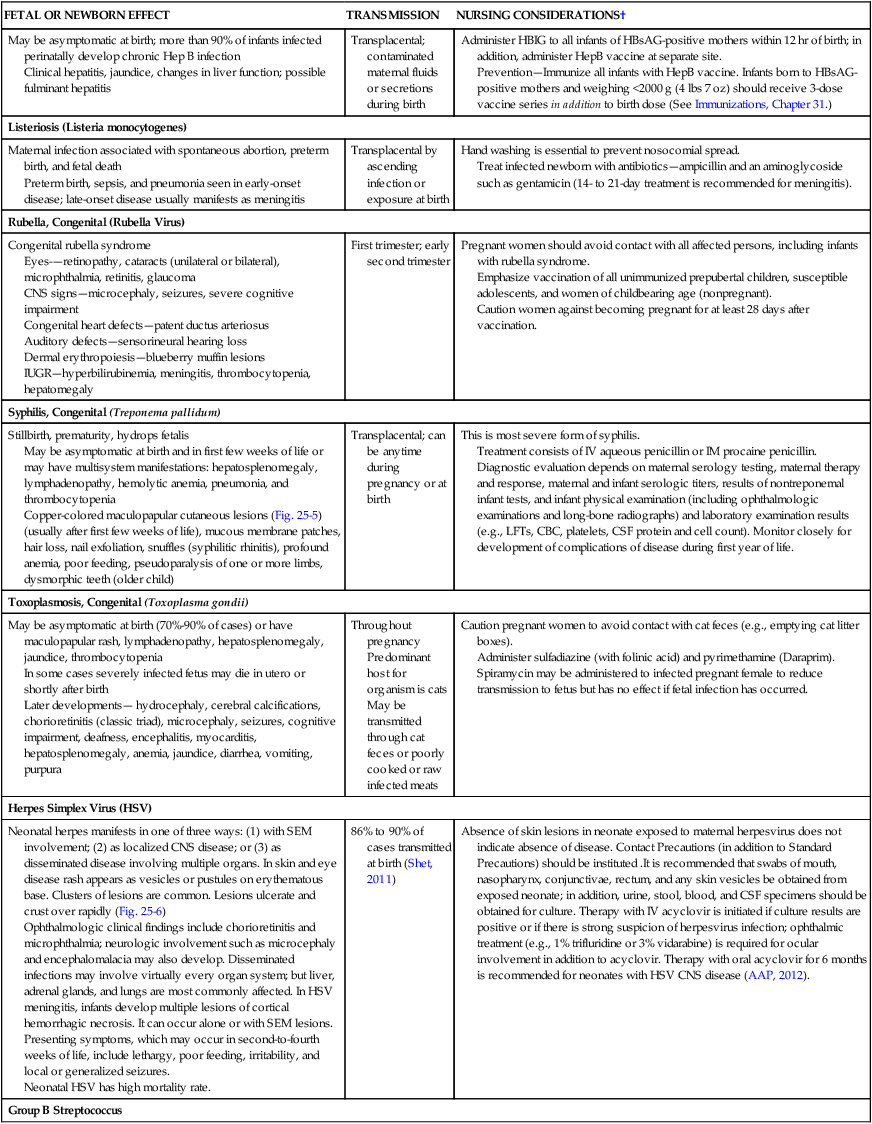
Hepatitis B Virus (HBV)
May be asymptomatic at birth; more than 90% of infants infected perinatally develop chronic Hep B infection
Clinical hepatitis, jaundice, changes in liver function; possible fulminant hepatitis
Transplacental; contaminated maternal fluids or secretions during birth
Administer HBIG to all infants of HBsAG-positive mothers within 12 hr of birth; in addition, administer HepB vaccine at separate site.
Prevention—Immunize all infants with HepB vaccine. Infants born to HBsAG-positive mothers and weighing <2000 g (4 lbs 7 oz) should receive 3-dose vaccine series in addition to birth dose (See Immunizations, Chapter 31.)
Listeriosis (Listeria monocytogenes)
Maternal infection associated with spontaneous abortion, preterm birth, and fetal death
Preterm birth, sepsis, and pneumonia seen in early-onset disease; late-onset disease usually manifests as meningitis
Transplacental by ascending infection or exposure at birth
Hand washing is essential to prevent nosocomial spread.
Treat infected newborn with antibiotics—ampicillin and an aminoglycoside such as gentamicin (14- to 21-day treatment is recommended for meningitis).
Rubella, Congenital (Rubella Virus)
Congenital rubella syndrome
Eyes-—retinopathy, cataracts (unilateral or bilateral), microphthalmia, retinitis, glaucoma
CNS signs—microcephaly, seizures, severe cognitive impairment
Congenital heart defects—patent ductus arteriosus
Auditory defects—sensorineural hearing loss
Dermal erythropoiesis—blueberry muffin lesions
IUGR—hyperbilirubinemia, meningitis, thrombocytopenia, hepatomegaly
First trimester; early second trimester
Pregnant women should avoid contact with all affected persons, including infants with rubella syndrome.
Emphasize vaccination of all unimmunized prepubertal children, susceptible adolescents, and women of childbearing age (nonpregnant).
Caution women against becoming pregnant for at least 28 days after vaccination.
Syphilis, Congenital (Treponema pallidum)
Stillbirth, prematurity, hydrops fetalis
May be asymptomatic at birth and in first few weeks of life or may have multisystem manifestations: hepatosplenomegaly, lymphadenopathy, hemolytic anemia, pneumonia, and thrombocytopenia
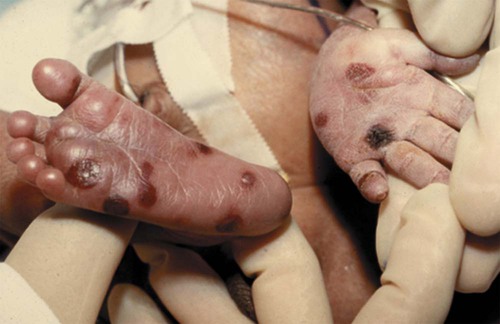
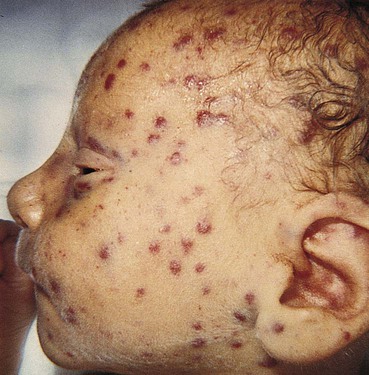
Copper-colored maculopapular cutaneous lesions (Fig. 25-5) (usually after first few weeks of life), mucous membrane patches, hair loss, nail exfoliation, snuffles (syphilitic rhinitis), profound anemia, poor feeding, pseudoparalysis of one or more limbs, dysmorphic teeth (older child)
Transplacental; can be anytime during pregnancy or at birth
This is most severe form of syphilis.
Treatment consists of IV aqueous penicillin or IM procaine penicillin.
Diagnostic evaluation depends on maternal serology testing, maternal therapy and response, maternal and infant serologic titers, results of nontreponemal infant tests, and infant physical examination (including ophthalmologic examinations and long-bone radiographs) and laboratory examination results (e.g., LFTs, CBC, platelets, CSF protein and cell count). Monitor closely for development of complications of disease during first year of life.
Toxoplasmosis, Congenital (Toxoplasma gondii)
May be asymptomatic at birth (70%-90% of cases) or have maculopapular rash, lymphadenopathy, hepatosplenomegaly, jaundice, thrombocytopenia
In some cases severely infected fetus may die in utero or shortly after birth
Later developments— hydrocephaly, cerebral calcifications, chorioretinitis (classic triad), microcephaly, seizures, cognitive impairment, deafness, encephalitis, myocarditis, hepatosplenomegaly, anemia, jaundice, diarrhea, vomiting, purpura
Throughout pregnancy
Predominant host for organism is cats
May be transmitted through cat feces or poorly cooked or raw infected meats
Caution pregnant women to avoid contact with cat feces (e.g., emptying cat litter boxes).
Administer sulfadiazine (with folinic acid) and pyrimethamine (Daraprim).
Spiramycin may be administered to infected pregnant female to reduce transmission to fetus but has no effect if fetal infection has occurred.
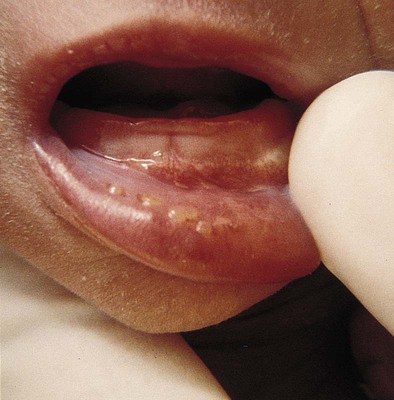
Herpes Simplex Virus (HSV)
Neonatal herpes manifests in one of three ways: (1) with SEM involvement; (2) as localized CNS disease; or (3) as disseminated disease involving multiple organs. In skin and eye disease rash appears as vesicles or pustules on erythematous base. Clusters of lesions are common. Lesions ulcerate and crust over rapidly (Fig. 25-6)
Ophthalmologic clinical findings include chorioretinitis and microphthalmia; neurologic involvement such as microcephaly and encephalomalacia may also develop. Disseminated infections may involve virtually every organ system; but liver, adrenal glands, and lungs are most commonly affected. In HSV meningitis, infants develop multiple lesions of cortical hemorrhagic necrosis. It can occur alone or with SEM lesions. Presenting symptoms, which may occur in second-to-fourth weeks of life, include lethargy, poor feeding, irritability, and local or generalized seizures.
Neonatal HSV has high mortality rate.
86% to 90% of cases transmitted at birth (Shet, 2011)
Absence of skin lesions in neonate exposed to maternal herpesvirus does not indicate absence of disease. Contact Precautions (in addition to Standard Precautions) should be instituted .It is recommended that swabs of mouth, nasopharynx, conjunctivae, rectum, and any skin vesicles be obtained from exposed neonate; in addition, urine, stool, blood, and CSF specimens should be obtained for culture. Therapy with IV acyclovir is initiated if culture results are positive or if there is strong suspicion of herpesvirus infection; ophthalmic treatment (e.g., 1% trifluridine or 3% vidarabine) is required for ocular involvement in addition to acyclovir. Therapy with oral acyclovir for 6 months is recommended for neonates with HSV CNS disease (AAP, 2012).
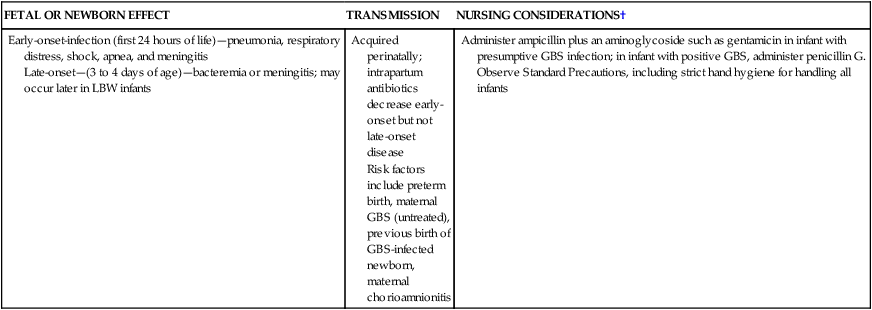
Group B Streptococcus
Early-onset-infection (first 24 hours of life)—pneumonia, respiratory distress, shock, apnea, and meningitis
Late-onset—(3 to 4 days of age)—bacteremia or meningitis; may occur later in LBW infants
Acquired perinatally; intrapartum antibiotics decrease early-onset but not late-onset disease
Risk factors include preterm birth, maternal GBS (untreated), previous birth of GBS-infected newborn, maternal chorioamnionitis
Administer ampicillin plus an aminoglycoside such as gentamicin in infant with presumptive GBS infection; in infant with positive GBS, administer penicillin G.
Observe Standard Precautions, including strict hand hygiene for handling all infants
The High Risk Newborn
Get Clinical Tree app for offline access