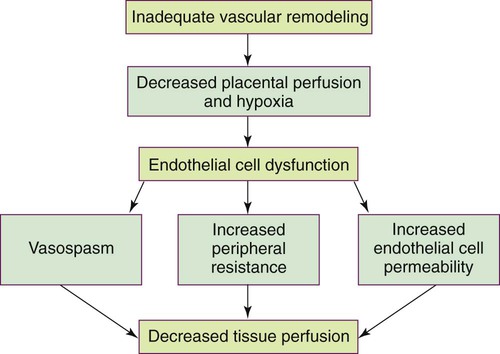
On completion of this chapter, the reader will be able to: • Differentiate among gestational hypertension, preeclampsia, and chronic hypertension. • Describe etiologic theories and pathophysiology of preeclampsia. • Compare care management of women with mild gestational hypertension or preeclampsia versus severe gestational hypertension or preeclampsia. • Discuss the preconception, antepartum, intrapartum, and postpartum management of the woman with chronic hypertension. • Explain the effects of hyperemesis gravidarum on maternal and fetal well-being. • Differentiate among causes, signs and symptoms, possible complications, and management of miscarriage, ectopic pregnancy, cervical insufficiency, and hydatidiform mole. • Compare and contrast placenta previa and placental abruption in relation to signs and symptoms, complications, and management. • Discuss the diagnosis and management of disseminated intravascular coagulation. • Discuss signs and symptoms, effects on pregnancy, and management of urinary tract infections. • Explain the basic principles of care for a pregnant woman undergoing abdominal surgery. • Discuss implications of trauma on mother and fetus during pregnancy. • Identify priorities in assessment and stabilization measures for the pregnant trauma victim. Hypertensive disorders are some of the most common medical complications of pregnancy, occurring in approximately 5% to 10% of all pregnancies. The incidence varies among hospitals, regions, and countries. Hypertensive disorders are a major cause of maternal and perinatal morbidity and mortality worldwide (Sibai, 2012). In the United States and Canada they are one of the top causes of maternal morbidity and mortality (Harvey and Sibai, 2013). The four most common types of hypertensive disorders occurring in pregnancy are: (1) gestational hypertension, (2) preeclampsia-eclampsia, (3) chronic hypertension, and (4) preeclampsia superimposed on chronic hypertension (Gilbert, 2011; Harvey and Sibai, 2013). The classification of hypertensive disorders in pregnancy is confusing because not all health care providers consistently use standard definitions. The classification system most commonly used in the United States is based on reports from the American College of Obstetricians and Gynecologists (ACOG, 2002) and the National High Blood Pressure Education Program (2000). This classification system is summarized in Table 12-1. TABLE 12-1 Classification of Hypertensive States of Pregnancy Data from American College of Obstetricians and Gynecologists (ACOG): Diagnosis and management of preeclampsia and eclampsia, AGOG Practice Bulletin No. 33, Washington DC, 2002, ACOG; Harvey C, Sibai B: Hypertension in pregnancy. In Troiano NH, Harvey CJ, Chez BF, editors: AWHONN’s high risk and critical care obstetrics, ed 3, Philadelphia, 2013, Lippincott Williams & Wilkins. Gestational hypertension is the onset of hypertension without proteinuria after week 20 of pregnancy (ACOG, 2002; National High Blood Pressure Education Program, 2000). Hypertension is defined as a systolic blood pressure (BP) greater than 140 mm Hg or a diastolic BP greater than 90 mm Hg. The hypertension should be recorded on at least two separate occasions at least 4 to 6 hours apart but within a maximum of a 1-week period (ACOG, 2002; Harvey and Sibai, 2013; National High Blood Pressure Education Program, 2000). Only one pressure (either systolic or diastolic) needs to be elevated to meet the definition of hypertension (Harvey and Sibai, 2013). Both the National High Blood Pressure Education Program and the American Heart Association (AHA) have published extensive recommendations for accurately measuring BP (Pickering, Hall, Appel, et al., 2005; National High Blood Pressure Education Program, 2000). Box 12-1 provides detailed instructions for measurement. Gestational hypertension is further classified as either mild or severe. The definitions of mild and severe gestational hypertension are the same as the definitions for BP readings for mild and severe preeclampsia (Table 12-2). Gestational hypertension does not persist longer than 12 weeks postpartum and usually resolves during the first postpartum week (Harvey and Sibai, 2013). Some women who initially are thought to have gestational hypertension will eventually be diagnosed with chronic hypertension. Others will develop proteinuria, thereby changing their diagnosis to preeclampsia. TABLE 12-2 Differentiation Between Mild And Severe Preeclampsia IUGR, Intrauterine growth restriction. Data from American College of Obstetricians and Gynecologists (ACOG): Diagnosis and management of preeclampsia and eclampsia, ACOG Practice Bulletin No. 33, Washington DC, 2002, ACOG; Harvey C, Sibai B: Hypertension in pregnancy. In Troiano NH, Harvey CJ, Chez BF, editors: AWHONN’s high risk and critical care obstetrics, ed 3, Philadelphia, 2013, Lippincott Williams & Wilkins. Preeclampsia is a pregnancy-specific condition in which hypertension and proteinuria develop after 20 weeks of gestation in a woman who previously had neither condition. It occurs in approximately 2% to 7% of healthy nulliparous pregnant women and much more frequently in women with multifetal gestation, a history of preeclampsia, chronic hypertension, and preexisting diabetes (Sibai, 2012). Preeclampsia is a vasospastic, systemic disorder and is usually categorized as mild or severe for purposes of management (ACOG, 2002; National High Blood Pressure Education Program, 2000). Table 12-2 lists criteria for mild and severe preeclampsia, and Table 12-3 gives common laboratory changes that occur in both. TABLE 12-3 Common Laboratory Changes In Preeclampsia *LDH values differ according to the test or assays being performed. Data from American College of Obstetricians and Gynecologists (ACOG): Diagnosis and management of preeclampsia and eclampsia, ACOG Practice Bulletin No. 33, Washington DC, 2002, ACOG; Cunningham F, Leveno K, Bloom S, et al, editors: Williams obstetrics, ed 23, New York, 2010, McGraw-Hill; Dildy G: Complications of preeclampsia. In Dildy G, Belfort M, Saade G, et al, editors: Critical care obstetrics, ed 4, Malden, MA, 2004, Blackwell Science; Harvey C, Sibai B: Hypertension in pregnancy. In Troiano NH, Harvey CJ, Chez BF, editors: AWHONN’s high risk and critical care obstetrics, ed 3, Philadelphia, 2013, Lippincott Williams & Wilkins. Eclampsia is the onset of seizure activity or coma in a woman with preeclampsia who has no history of preexisting pathology that can result in seizure activity (Harvey and Sibai, 2013; Roberts and Funai, 2009). Eclamptic seizures can occur before, during, or after birth. Approximately one third of eclamptic seizures occur after birth, almost always within the first 48 hours after birth (Roberts and Funai, 2009). Chronic hypertension is defined as hypertension that is present before the pregnancy or develops before 20 weeks of gestation. Hypertension initially diagnosed during pregnancy that persists longer than 12 weeks postpartum is also classified as chronic hypertension (Harvey and Sibai, 2013; Roberts and Funai, 2009). Chronic hypertension is further classified as mild or severe based on systolic and diastolic BPs. Most women with mild chronic hypertension experience uncomplicated pregnancies. However, those with severe chronic hypertension have an increased risk of perinatal mortality (Gilbert, 2011). Women with chronic hypertension may develop superimposed preeclampsia, which increases the morbidity for mother and fetus. A diagnosis of chronic hypertension with superimposed preeclampsia is made with the following findings (Harvey and Sibai, 2013): Preeclampsia is a condition unique to human pregnancy. Signs and symptoms develop only during pregnancy and disappear soon after birth of the fetus and expulsion of the placenta. Common risk factors associated with the development of preeclampsia are listed in Box 12-2. The strongest risk factors are a first pregnancy when the woman is younger than 19 or older than 40 years of age, a first pregnancy with a new partner, and a history of severe preeclampsia (Gilbert, 2011). The cause of preeclampsia is unknown. Many theories have been suggested to explain its etiology. Current theories that are still being considered include abnormal trophoblast invasion, immunologic response to partially foreign genetic placental and fetal tissue, stimulation of the inflammatory system by cardiovascular changes of pregnancy, various dietary deficiencies, and genetic abnormalities (Harvey and Sibai, 2013). Preeclampsia can progress along a continuum from mild-to-severe preeclampsia to eclampsia. Current thought is that the pathologic changes that occur in the woman with preeclampsia are caused by disruptions in placental perfusion and endothelial cell dysfunction (Gilbert, 2011; Harvey and Sibai, 2013; Sibai, 2012). These changes are present long before the clinical diagnosis of preeclampsia is made (Roberts and Funai, 2009). Normally in pregnancy the spiral arteries in the uterus widen from thick-walled muscular vessels to thinner, saclike vessels with much larger diameters. This change increases the capacity of the vessels, allowing them to handle the increased blood volume of pregnancy. Because this vascular remodeling does not occur or only partially develops in women with preeclampsia, decreased placental perfusion and hypoxia result (Harvey and Sibai, 2013). Placental ischemia is thought to cause endothelial cell dysfunction by stimulating the release of a substance that is toxic to endothelial cells. This anomaly causes generalized vasospasm, which results in poor tissue perfusion in all organ systems, increased peripheral resistance and BP, and increased endothelial cell permeability, leading to intravascular protein and fluid loss and ultimately to less plasma volume. The main pathogenic factor is not an increase in BP but poor perfusion as a result of vasospasm and reduced plasma volume (Fig. 12-1) (Gilbert, 2011; Roberts and Funai, 2009). Fig. 12-2 demonstrates how endothelial cell dysfunction causes many of the common signs and symptoms of preeclampsia. Reduced kidney perfusion decreases the glomerular filtration rate and can lead to degenerative glomerular changes and oliguria. Pathologic changes in the endothelial cells of the glomeruli (glomerular endotheliosis) are uniquely characteristic of preeclampsia. Protein, primarily albumin, is lost in the urine. Uric acid clearance is decreased, but serum uric acid levels increase. Sodium and water are retained. Acute tubular necrosis and renal failure may occur (Gilbert, 2011; Roberts and Funai, 2009). Plasma colloid osmotic pressure decreases as serum albumin levels decrease. Intravascular volume is reduced as fluid moves out of the intravascular compartment, resulting in hemoconcentration, increased blood viscosity, and tissue edema. The hematocrit value increases as fluid leaves the intravascular space. Arteriolar vasospasm can lead to endothelial damage and increased capillary permeability, predisposing the woman to pulmonary edema (see Fig. 12-2) (Gilbert, 2011; Roberts and Funai, 2009). Decreased liver perfusion can lead to impaired liver function and elevated liver enzyme levels. If hepatic edema and subcapsular hemorrhage develop, the woman may complain of epigastric or right upper quadrant pain. Hemorrhagic necrosis in the liver can result in a subcapsular hematoma, which is a rare occurrence (Gilbert, 2011). Rupture of a subcapsular hematoma is a life-threatening complication and a surgical emergency (see Fig. 12-2). Neurologic complications associated with preeclampsia include cerebral edema and hemorrhage and increased central nervous system (CNS) irritability. CNS irritability manifests as headaches, hyperreflexia, positive ankle clonus, and seizures. Arteriolar vasospasms and decreased blood flow to the retina can lead to visual disturbances such as scotoma (dim vision or blind or dark spots in the visual field) and blurred or double vision (Gilbert, 2011; Roberts and Funai, 2009). Traditionally preeclampsia was considered to affect the woman primarily, causing hypertension, proteinuria, and perhaps associated multisystem dysfunction. However, currently it is recognized that it can also primarily affect the fetus, resulting in fetal growth restriction, decreased amniotic fluid volume, abnormal fetal oxygenation, low birth weight, and preterm birth (Sibai, 2012). HELLP syndrome is a laboratory diagnosis for a variant of severe preeclampsia that involves hepatic dysfunction, characterized by hemolysis (H), elevated liver enzymes (EL), and low platelet count (LP). It is not a separate illness. Specific laboratory findings are needed to diagnose HELLP syndrome and distinguish it from other serious diseases that share the same signs and symptoms. HELLP syndrome occurs in 0.5% to 0.9% of all pregnancies. Ten to twenty percent of women with severe preeclampsia develop it (Harvey and Sibai, 2013). Table 12-3 lists laboratory changes that occur in HELLP syndrome. Traditionally it was not diagnosed unless all three laboratory abnormalities were present. Recently, however, women who develop only one or two of the diagnostic laboratory values are being diagnosed with incomplete HELLP, partial HELLP, or the ELLP syndrome (Harvey and Sibai, 2013). The pathophysiologic changes of HELLP syndrome occur as a result of arteriolar vasospasm, endothelial cell dysfunction with fibrin deposits, and adherence of platelets in blood vessels. Red blood cells are damaged as they pass through narrowed blood vessels and become hemolyzed, resulting in a decreased red blood cell and platelet count and hyperbilirubinemia. Endothelial damage and fibrin deposits in the liver lead to impaired liver function and can cause hemorrhagic necrosis. Liver enzymes are elevated when hepatic tissue is damaged (Gilbert, 2011). HELLP syndrome usually develops during the antepartum period. The clinical presentation is often nonspecific. Most women with the disorder report a history of malaise, influenza-like symptoms, and epigastric or right upper quadrant abdominal pain. Symptoms tend to worsen at night and improve during the daytime. HELLP syndrome can progress rapidly (Harvey and Sibai, 2013). HELLP syndrome appears to occur more frequently in Caucasian women than women of other races. A diagnosis of HELLP syndrome is associated with an increased risk for maternal death and adverse perinatal outcomes, including pulmonary edema, acute renal failure, disseminated intravascular coagulopathy (DIC), placental abruption, liver hemorrhage or failure, acute respiratory distress syndrome (ARDS), sepsis, and stroke (Sibai, 2012). The reported perinatal mortality rate ranges from 7.4% to 34%, with a maternal mortality rate of approximately 1% (Sibai, 2012). The rate of preterm birth in women with HELLP syndrome is approximately 70%, with 15% of these occurring before 28 weeks of gestation. Most of the perinatal deaths occur before 28 weeks of gestation in association with placental abruption or severe fetal growth restriction (Sibai, 2012). Numerous clinical trials have examined various interventions to prevent preeclampsia, including protein or salt restriction; zinc, magnesium, fish oil, or vitamins C and E supplementation; use of diuretics or other antihypertensive medications; and use of heparin or low dose aspirin. All of these interventions demonstrated minimal to no benefit in preventing or reducing the severity of preeclampsia (Sibai, 2012). No reliable test that can be used as a routine screening tool for predicting preeclampsia has yet been developed. However, the search for biomarkers that can identify individual women who will develop hypertension during pregnancy is ongoing (Harvey and Sibai, 2013). For example, the tyrosine kinase (sFLt) and serum placental growth factor (PIGF) ratio at 22 to 26 weeks of gestation was shown in one study to be highly predictive of early-onset preeclampsia (Gilbert, 2011). An abnormal uterine artery Doppler velocimetry in the first or second trimester of pregnancy has also been suggested as a good screening test to predict preeclampsia (Gilbert, 2011).
High Risk Perinatal Care
Gestational Conditions
Hypertension in Pregnancy
Significance and Incidence
Classification
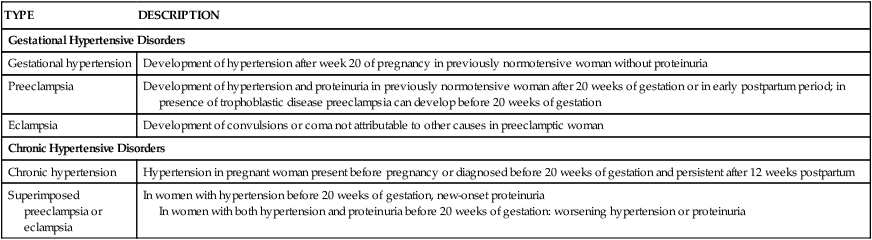
TYPE
DESCRIPTION
Gestational Hypertensive Disorders
Gestational hypertension
Development of hypertension after week 20 of pregnancy in previously normotensive woman without proteinuria
Preeclampsia
Development of hypertension and proteinuria in previously normotensive woman after 20 weeks of gestation or in early postpartum period; in presence of trophoblastic disease preeclampsia can develop before 20 weeks of gestation
Eclampsia
Development of convulsions or coma not attributable to other causes in preeclamptic woman
Chronic Hypertensive Disorders
Chronic hypertension
Hypertension in pregnant woman present before pregnancy or diagnosed before 20 weeks of gestation and persistent after 12 weeks postpartum
Superimposed preeclampsia or eclampsia
In women with hypertension before 20 weeks of gestation, new-onset proteinuria
In women with both hypertension and proteinuria before 20 weeks of gestation: worsening hypertension or proteinuria
Gestational Hypertension
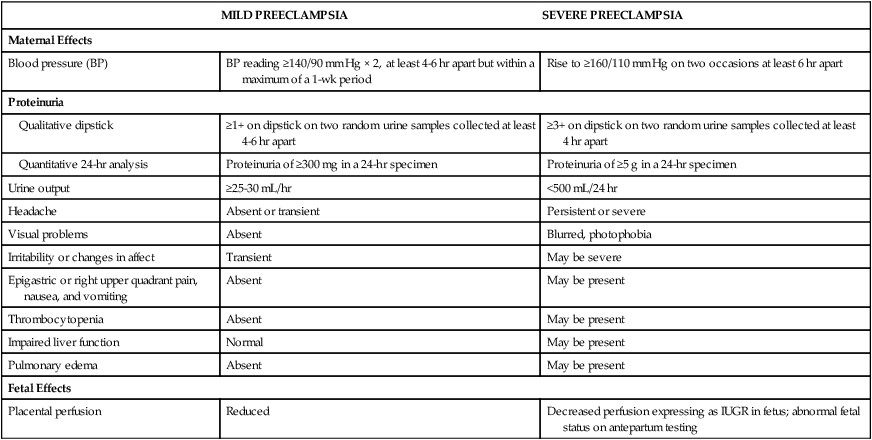
MILD PREECLAMPSIA
SEVERE PREECLAMPSIA
Maternal Effects
Blood pressure (BP)
BP reading ≥140/90 mm Hg × 2, at least 4-6 hr apart but within a maximum of a 1-wk period
Rise to ≥160/110 mm Hg on two occasions at least 6 hr apart
Proteinuria
Qualitative dipstick
≥1+ on dipstick on two random urine samples collected at least 4-6 hr apart
≥3+ on dipstick on two random urine samples collected at least 4 hr apart
Quantitative 24-hr analysis
Proteinuria of ≥300 mg in a 24-hr specimen
Proteinuria of ≥5 g in a 24-hr specimen
Urine output
≥25-30 mL/hr
<500 mL/24 hr
Headache
Absent or transient
Persistent or severe
Visual problems
Absent
Blurred, photophobia
Irritability or changes in affect
Transient
May be severe
Epigastric or right upper quadrant pain, nausea, and vomiting
Absent
May be present
Thrombocytopenia
Absent
May be present
Impaired liver function
Normal
May be present
Pulmonary edema
Absent
May be present
Fetal Effects
Placental perfusion
Reduced
Decreased perfusion expressing as IUGR in fetus; abnormal fetal status on antepartum testing
Preeclampsia
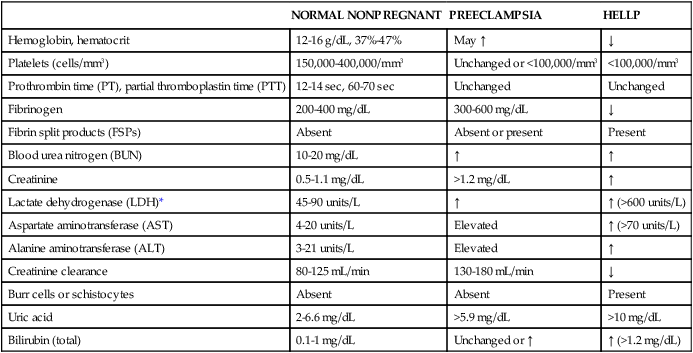
NORMAL NONPREGNANT
PREECLAMPSIA
HELLP
Hemoglobin, hematocrit
12-16 g/dL, 37%-47%
May ↑
↓
Platelets (cells/mm3)
150,000-400,000/mm3
Unchanged or <100,000/mm3
<100,000/mm3
Prothrombin time (PT), partial thromboplastin time (PTT)
12-14 sec, 60-70 sec
Unchanged
Unchanged
Fibrinogen
200-400 mg/dL
300-600 mg/dL
↓
Fibrin split products (FSPs)
Absent
Absent or present
Present
Blood urea nitrogen (BUN)
10-20 mg/dL
↑
↑
Creatinine
0.5-1.1 mg/dL
>1.2 mg/dL
↑
Lactate dehydrogenase (LDH)*
45-90 units/L
↑
↑ (>600 units/L)
Aspartate aminotransferase (AST)
4-20 units/L
Elevated
↑ (>70 units/L)
Alanine aminotransferase (ALT)
3-21 units/L
Elevated
↑
Creatinine clearance
80-125 mL/min
130-180 mL/min
↓
Burr cells or schistocytes
Absent
Absent
Present
Uric acid
2-6.6 mg/dL
>5.9 mg/dL
>10 mg/dL
Bilirubin (total)
0.1-1 mg/dL
Unchanged or ↑
↑ (>1.2 mg/dL)
Eclampsia
Chronic Hypertensive Disorders
Chronic Hypertension.
Chronic Hypertension with Superimposed Preeclampsia.
Preeclampsia
Etiology
Pathophysiology
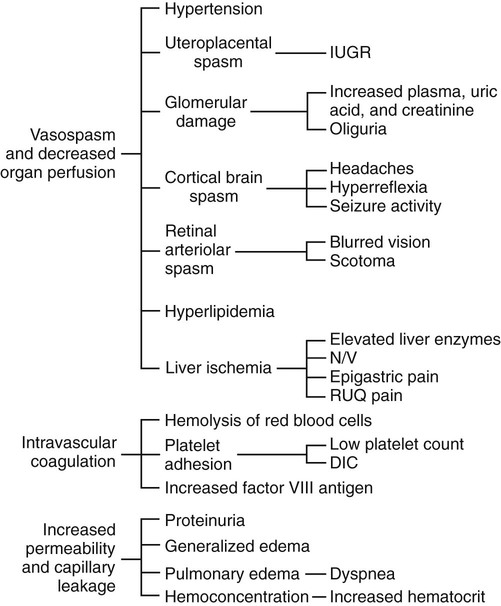
HELLP Syndrome
Care Management
Identifying and Preventing Preeclampsia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


High Risk Perinatal Care: Gestational Conditions
Get Clinical Tree app for offline access
