(fos-KAR-net SO-dee-um)
Foscavir
Antiviral
pH 7.4
Usual dose
An individualized dose should be calculated based on body weight (mg/kg), renal function, indication of use, and dosing frequency. To reduce the risk of nephrotoxicity, creatinine clearance (mL/min/kg) should be calculated even if SCr is within the normal range. Adequate hydration and other specific testing required; see Monitor.
CMV retinitis:
90 mg/kg every 12 hours or 60 mg/kg every 8 hours for 14 to 21 days. Length of induction treatment based on clinical response. Begin a maintenance dose of 90 mg/kg/day the next day (Day 15 to 22). If retinitis progresses during the maintenance regimen, re-treat with the induction and maintenance regimens. Maintenance dose may be increased to 120 mg/kg/day in patients who show excellent tolerance to foscarnet or those who require early re-induction because of retinitis progression. Normal renal function required. For patients who have relapsed after induction and re-induction monotherapy with either foscarnet or ganciclovir, practitioners may consider combination therapy, which adds the alternate drug to the regimen. See the Clinical Trials section of the foscarnet sodium prescribing information.
Acyclovir-resistant HSV patients:
40 mg/kg every 8 or 12 hours for 2 to 3 weeks or until healed.
Dose adjustments
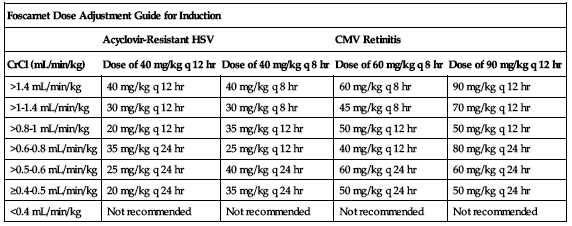
Must be reduced and individualized according to patient’s renal function. Safety and efficacy data for patients with baseline SCr greater than 2.8 mg/dL or measured 24-hour CrCl less than 50 mL/min are limited. Dose adjustment may be required during treatment even if patient had normal renal function initially. Specific calculation and testing are required for both induction and maintenance dose. Specific calculation for induction is shown in the following chart.
| Foscarnet Dose Adjustment Guide for Induction | ||||
| Acyclovir-Resistant HSV | CMV Retinitis | |||
| CrCl (mL/min/kg) | Dose of 40 mg/kg q 12 hr | Dose of 40 mg/kg q 8 hr | Dose of 60 mg/kg q 8 hr | Dose of 90 mg/kg q 12 hr |
| >1.4 mL/min/kg | 40 mg/kg q 12 hr | 40 mg/kg q 8 hr | 60 mg/kg q 8 hr | 90 mg/kg q 12 hr |
| >1-1.4 mL/min/kg | 30 mg/kg q 12 hr | 30 mg/kg q 8 hr | 45 mg/kg q 8 hr | 70 mg/kg q 12 hr |
| >0.8-1 mL/min/kg | 20 mg/kg q 12 hr | 35 mg/kg q 12 hr | 50 mg/kg q 12 hr | 50 mg/kg q 12 hr |
| >0.6-0.8 mL/min/kg | 35 mg/kg q 24 hr | 25 mg/kg q 12 hr | 40 mg/kg q 12 hr | 80 mg/kg q 24 hr |
| >0.5-0.6 mL/min/kg | 25 mg/kg q 24 hr | 40 mg/kg q 24 hr | 60 mg/kg q 24 hr | 60 mg/kg q 24 hr |
| ≥0.4-0.5 mL/min/kg | 20 mg/kg q 24 hr | 35 mg/kg q 24 hr | 50 mg/kg q 24 hr | 50 mg/kg q 24 hr |
| <0.4 mL/min/kg | Not recommended | Not recommended | Not recommended | Not recommended |
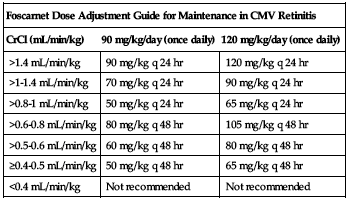
Specific calculation for maintenance dose is shown in the following chart.
| Foscarnet Dose Adjustment Guide for Maintenance in CMV Retinitis | ||
| CrCl (mL/min/kg) | 90 mg/kg/day (once daily) | 120 mg/kg/day (once daily) |
| >1.4 mL/min/kg | 90 mg/kg q 24 hr | 120 mg/kg q 24 hr |
| >1-1.4 mL/min/kg | 70 mg/kg q 24 hr | 90 mg/kg q 24 hr |
| >0.8-1 mL/min/kg | 50 mg/kg q 24 hr | 65 mg/kg q 24 hr |
| >0.6-0.8 mL/min/kg | 80 mg/kg q 48 hr | 105 mg/kg q 48 hr |
| >0.5-0.6 mL/min/kg | 60 mg/kg q 48 hr | 80 mg/kg q 48 hr |
| ≥0.4-0.5 mL/min/kg | 50 mg/kg q 48 hr | 65 mg/kg q 48 hr |
| <0.4 mL/min/kg | Not recommended | Not recommended |
Foscarnet is not recommended in patients undergoing hemodialysis because dosage guidelines have not been established.
Dilution
An individualized dose at the required concentration (24 mg/mL or 12 mg/mL) for the route administration (central line or peripheral line) must be aseptically prepared before dispensing. Remove any excess quantity of medication from the infusion bottle using aseptic technique. To avoid any possibility of overdose, only the calculated dose should be in the infusion bottle. Discard any excess before administration.
Central line:
Standard 24-mg/mL solution may be given undiluted.
Peripheral line:
Each 1 mL of a calculated dose must be diluted with 1 mL of D5W or NS (yields a 12-mg/mL solution).
Storage:
Store at CRT. Avoid excessive heat and freezing. Use only if vacuum is present and solution is clear and colorless. Use solution within 24 hours of entry into bottle.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Manufacturer states, “Administer only with NS or D5W solutions; no other drug or supplement should be administered concurrently via the same catheter.” Because of chelating properties, a precipitate can occur. Manufacturer specifically lists as incompatible with 30% dextrose, solutions containing calcium (e.g., LR, TPN), acyclovir (Zovirax), amphotericin B (conventional), diazepam (Valium), digoxin (Lanoxin), ganciclovir (Cytovene IV), leucovorin calcium, midazolam (Versed), pentamidine, phenytoin (Dilantin), prochlorperazine (Compazine), sulfamethoxazole/trimethoprim, vancomycin.
One source suggests the following compatibilities:
Additive:
Not recommended by manufacturer.
Potassium chloride (KCl).
Y-site:
Not recommended by manufacturer.
Aldesleukin (Proleukin), amikacin, aminophylline, ampicillin, aztreonam (Azactam), cefazolin (Ancef), cefoxitin (Mefoxin), ceftazidime (Fortaz), ceftriaxone (Rocephin), cefuroxime (Zinacef), chloramphenicol (Chloromycetin), clindamycin (Cleocin), dexamethasone (Decadron), dopamine, doripenem (Doribax), erythromycin (Erythrocin), fluconazole (Diflucan), furosemide (Lasix), gentamicin, heparin, hydrocortisone sodium succinate (Solu-Cortef), hydromorphone (Dilaudid), imipenem-cilastatin (Primaxin), lorazepam (Ativan), metoclopramide (Reglan), metronidazole (Flagyl IV), morphine, nafcillin (Nallpen), oxacillin (Bactocill), penicillin G potassium, ranitidine (Zantac), sulfamethoxazole/trimethoprim, ticarcillin/clavulanate (Timentin), tobramycin, vancomycin.
Rate of administration
Infusion pump required to deliver accurate dose evenly distributed over specific time frame. Excessive plasma levels and toxicity (including hypocalcemia) will occur with too-rapid rate of infusion. Advisable to clear tubing with NS if possible before and after administration through Y-tube or three-way stopcock. Never exceed 1 mg/kg/min.
CMV retinitis:
Induction doses:
Each 60-mg/kg dose equally distributed over a minimum of 1 hour. Increase to 11/2 to 2 hours for 90-mg/kg dose.
Maintenance dose:
Each dose equally distributed over a minimum of 2 hours.
Acyclovir-resistant HSV:
Each dose equally distributed over a minimum of 1 hour.
Actions
An antiviral agent capable of inhibiting replication of herpesviruses, including cytomegalovirus (CMV), herpes simplex virus types 1 and 2 (HSV-1, HSV-2), and varicella-zoster virus (VZV [unlabeled]). Does not destroy existing viruses but stops them from reproducing and invading healthy cells. Also capable of chelating metal ions (e.g., calcium, magnesium). CMV strains resistant to ganciclovir and HSV strains resistant to acyclovir may be sensitive to foscarnet. Some penetration into bone and cerebrospinal fluid. Plasma half-life ranges from 2 to 6 hours and increases markedly with renal impairment. Terminal half-life determined by urinary excretion was 87.5 +/− 41.8 hours, possibly due to release of foscarnet from bone. Excreted unchanged in urine.
Indications and uses
Treatment of CMV retinitis in patients with AIDS. Most frequently used in patients who do not tolerate or are resistant to ganciclovir. Combination therapy with ganciclovir is indicated for patients who have relapsed after monotherapy with either drug. ■ Treatment of acyclovir-resistant mucocutaneous HSV infections in immunocompromised patients.
Contraindications
Hypersensitivity to foscarnet.
Precautions
For IV use only. ■ Safety and efficacy of foscarnet have not been established for treatment of other CMV infections (e.g., pneumonitis, gastroenteritis), congenital or neonatal CMV disease, other HSV infections (e.g., retinitis, encephalitis), congenital or neonatal HSV disease, or CMV or HSV in nonimmunocompromised individuals. Use should be limited to treatment of conditions listed in Indications and Uses above. ■ May cause potentially life-threatening changes in renal function with cumulative exposure. Careful monitoring of renal function and dose adjustment is imperative. Changes can occur at any time, most likely during second week of therapy. Elevations in SCr are usually reversible following dose adjustment or discontinuation of therapy. ■ Confirm diagnosis of CMV retinitis by indirect ophthalmoscopy. Diagnosis may be supported by cultures of CMV from the throat and body fluids such as urine and blood; negative culture does not rule out CMV retinitis. ■ Use caution in patients with a history of impaired renal function, altered calcium or other electrolyte levels, neurologic or cardiac abnormalities, a low baseline absolute neutrophil count (ANC), and those receiving other drugs known to influence minerals and electrolytes; see Drug/Lab Interactions. Has caused hyperphosphatemia, hypocalcemia, hypokalemia, hypomagnesemia, and hypophosphatemia, resulting in cardiac disturbances, seizures, and tetany. ■ Seizures related to alterations in plasma minerals and electrolytes have been reported. Several cases were associated with death. Risk factors for seizures included impaired baseline renal function, low total serum calcium, and underlying CNS conditions. ■ Contains 5.5 mg sodium/mL. Avoid use in patients who may not tolerate a large amount of sodium or water (e.g., patients with cardiomyopathy) or in patients on a controlled-sodium diet. ■ Anemia has been reported in 33% of patients receiving foscarnet. ■ Resistance has been reported to develop. May be higher in patients treated for a prolonged period. Consider the possibility of resistance in patients who show poor clinical response or who experience persistent viral excretion during treatment. ■ Amino acid substitutions conferring foscarnet resistance with cross-resistance to ganciclovir, cidofovir, and acyclovir have been identified. ■ Sensitivity testing of the viral isolate is recommended before repeat treatment and/or to evaluate sensitivity versus development of resistance.
Monitor:
Baseline 24-hour CrCl verified by creatinine index; baseline SCr, calcium, magnesium, potassium, phosphorus, and electrolytes required before treatment begins. Correct any deficiencies. ■ Repeat entire testing process 2 to 3 times a week during induction therapy and weekly during maintenance therapy. Foscarnet dose must be adjusted as indicated by test results. More frequent testing may be indicated in specific patients. Supplementation of minerals and electrolytes may be required during treatment. ■ To minimize renal toxicity, hydration adequate to establish diuresis is recommended before and during treatment. Clinically dehydrated patients should be adequately hydrated before initiating therapy. Give 750 to 1,000 mL NS or D5W before the first foscarnet infusion to establish diuresis. With subsequent infusions, give 750 to 1,000 mL concurrently with 90 to 120 mg/kg foscarnet and a minimum of 500 mL concurrently with 40 to 60 mg/kg. Oral rehydration may be considered in certain patients. Hydration fluid may need to be decreased if clinically warranted. ■ Discontinue foscarnet if CrCl drops below 0.4 mL/min/kg. Monitor patient daily until resolution of renal impairment is ensured. Safety for use in these patients has not been studied. ■ Anemia may be severe enough to require transfusion. ■ Phlebitis or pain may occur at site of infusion; confirm patency of vein and use large veins to ensure adequate blood flow for rapid dilution and distribution. ■ See Drug/Lab Interactions.
Patient education:
Not a cure. Retinitis may recur during maintenance or after treatment; regular ophthalmologic exams imperative. ■ Complete healing of HSV infections may occur, but most relapse. ■ Perioral tingling, numbness in the extremities or paresthesias indicate electrolyte abnormalities; report immediately. ■ Close monitoring of renal function and electrolyte balance during treatment is imperative. ■ Cases of male and female genital irritation/ulceration have been reported. Adequate hydration and increased personal hygiene may minimize some side effects. ■ May cause CNS side effects (e.g., dizziness, seizures, somnolence) that could result in impairment. Use caution when driving or operating machinery. ■ Dose modification or discontinuation may be required for major side effects.
Maternal/child:
Category C: use only if clearly needed; has caused skeletal anomalies in animals. ■ Discontinue breast-feeding. ■ Safety for use in pediatric patients not established; deposited in teeth and bones, and deposition greater in young animals. Use only if benefits outweigh risks.
Elderly:
Safety and effectiveness not established; however, foscarnet has been used in patients over 65 years of age. Side effects seen are similar to other age-groups. Consider age-related renal impairment in dose selection, and monitor renal function; see Dose Adjustments.
Drug/lab interactions
Because of physical incompatibilities, foscarnet sodium and ganciclovir sodium must never be mixed. ■ Has caused hypocalcemia with parenteral pentamidine; deaths have been reported. ■ Capable of causing calcium or electrolyte disorders. Use particular caution when administering any drug known to influence serum calcium concentrations, other minerals, or electrolytes (e.g., hypocalcemic agents [gallium nitrate (Ganite)], diuretics [furosemide (Lasix), mannitol], adrenocortical steroids). ■ Elimination of foscarnet may be impaired and toxicity increased by drugs that inhibit renal tubular secretion. When diuretics are indicated, thiazides (e.g., hydrochlorothiazide) are recommended over loop diuretics (e.g., furosemide [Lasix]) because the latter inhibit renal tubular secretion. ■ Avoid concomitant use with other nephrotoxic drugs (e.g., acyclovir [Zovirax], aminoglycosides [e.g., gentamicin], amphotericin B [conventional], cyclosporine [Sandimmune], methotrexate, pentamidine, tacrolimus [Prograf]) unless benefits outweigh risks. ■ Use with fluoroquinolones (e.g., ciprofloxacin [Cipro]) may increase the risk of seizures; monitor patient carefully. ■ Abnormal renal function has been reported in patients receiving foscarnet and ritonavir (Norvir) or foscarnet and ritonavir and saquinavir (Invirase). ■ There is no clinically significant interaction with zidovudine (AZT) or probenecid. ■ The combination antiviral activity of foscarnet and ganciclovir or acyclovir is not antagonistic in cell culture.
Side effects
Impaired renal function, alterations in plasma minerals and electrolytes, and seizures are major side effects and are dose limiting. Abnormal renal function, including acute renal failure, decreased CrCl, and increased SCr; anemia; bone marrow suppression; diarrhea; fatigue; fever; headache; hyperphosphatemia; hypocalcemia (perioral tingling, numbness in extremities, paresthesias, tetany); hypokalemia; hypomagnesemia; hypophosphatemia; irritation at injection site; irritation and ulcerations of penile and vaginal epithelium; nausea; rigors; seizure; vomiting; and death have occurred. All deaths could not be directly related to foscarnet. Abdominal pain, anorexia, anxiety, asthenia, confusion, coughing, depression, dizziness, dyspnea, granulocytopenia, hypoesthesia, infection, involuntary muscle contractions, leukopenia, malaise, neuropathy, pain, rash, sweating, and vision abnormalities have occurred in 5% of patients. Numerous other side effects have occurred in fewer than 5% of patients.
Post-marketing:
Crystal-induced nephropathy, esophageal ulcerations, renal tubular acidosis, renal tubular necrosis, muscle disorders (e.g., myopathy, myositis, muscle weakness, rhabdomyolysis), vesiculobullous eruptions (e.g., erythema multiforme, toxic epidermal necrolysis, Stevens-Johnson syndrome).
Antidote
There is no specific antidote. Keep physician informed. Adequate hydration and careful monitoring will help reduce potential for renal impairment and may minimize other side effects. Elevations in SCr are usually reversible (within 1 week) with dose adjustment or discontinuation but have caused death. Discontinue foscarnet if CrCl falls below 0.4 mL/min/kg. Monitor daily until resolution of renal impairment is ensured. Discontinue foscarnet if perioral tingling, numbness in the extremities, or paresthesias occur during or after infusion; evaluate calcium and electrolyte levels (decrease in ionized serum calcium may not be reflected in total serum calcium); notify physician. Administration of foscarnet can be resumed following seizures or cardiac disturbances after treatment of underlying disease, electrolyte disturbance, or after dose adjustment. Overdose can occur with too-rapid rate of infusion. Hemodialysis and hydration may be useful in overdose. Treat anaphylaxis and resuscitate as indicated.
Fosphenytoin sodium 
(FOS-fen-ih-toyn SO-dee-um)
Cerebyx
Anticonvulsant (hydantoin)
pH 8.6 to 9
Usual dose
1.5 mg of fosphenytoin sodium is equivalent to 1 mg phenytoin sodium and is referred to as 1 mg phenytoin sodium equivalents (PE). The amount and concentration of fosphenytoin is always expressed in terms of mg PE. Fosphenytoin 15 to 20 mg PE/kg is equivalent to phenytoin 15 to 20 mg/kg, or a 1,000-mg PE dose of fosphenytoin is equivalent to a 1,000-mg dose of phenytoin. Because of the risk of hypotension and cardiac arrhythmias, fosphenytoin should be administered no faster than 150 mg PE/min; see Rate of Administration, Precautions, and Monitor.
Status epilepticus:
Adult loading dose:
15 to 20 mg PE/kg. Full effect is not immediate; concomitant administration of an IV benzodiazepine (e.g., diazepam [Valium]) is usually necessary to control status epilepticus. If seizures are not controlled, consider other anticonvulsants and other measures as needed (e.g., barbiturates or anesthesia).
Maintenance dose:
4 to 6 mg PE/kg/24 hr in divided doses.
Another source adds: Elderly loading dose: 14 mg PE/kg. See all comments under Usual Dose. See Elderly.
Nonemergent indications:
Loading dose:
10 to 20 mg PE/kg.
Maintenance dose:
4 to 6 mg PE/kg/24 hr in divided doses. Because of the risk of cardiac and local toxicity associated with IV fosphenytoin, oral phenytoin should be administered whenever possible.
Substitute for oral phenytoin:
May be substituted at the same total daily phenytoin sodium equivalents (PE) dose (due to a 10% increase in bioavailability [IV/IM to oral], plasma levels with the IV/IM product may be increased slightly).
Pediatric dose
See comments in Usual Dose.
Status epilepticus loading dose (unlabeled):
15 to 20 mg PE/kg at a rate of 3 mg PE/kg/min; do not exceed 150 mg PE/min.
Nonemergent loading dose:
10 to 20 mg PE/kg.
Maintenance dose:
4 to 6 mg PE/kg/24 hr initially; may be given as a single dose or divided into 2 doses. Safety for use in pediatric patients not established. Limited data available; no significant differences apparent to this date; see Maternal/Child.
Dose adjustments
Reduced doses may be required in the elderly (see Usual Dose), in impaired renal or hepatic function, or in patients with hypoalbuminemia. ■ See Precautions, Monitor, Drug/Lab Interactions, and Antidote.
Dilution
Use only clear solutions. Should be diluted in D5W or NS to a concentration of 1.5 to 25 mg PE/mL. Supplied solution is 50 mg PE/mL. Do not confuse the amount of drug to be given in PE with the concentration of the drug in the vial; serious errors have occurred. Dilute each milliliter of fosphenytoin with 1 mL of diluent to equal 25 mg PE/mL. Dilute a 1,000-mg PE dose in 100 mL diluent to equal 10 mg PE/mL.
Storage:
Keep refrigerated; do not store at room temperature for more than 48 hours. For single use only. Discard any unused product after opening.
Compatibility
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
One source suggests the following compatibilities:
Additive:
Hetastarch in NS (Hespan), mannitol, potassium chloride (KCl).
Y-site:
Rate of administration
Each 100 to 150 mg PE or fraction thereof over a minimum of 1 minute. Do not exceed a rate of 150 mg PE/min. Risk of severe hypotension and cardiac arrhythmias increased. Slow or temporarily stop rate of infusion for cardiovascular side effects or for burning, itching, numbness, or pain along injection site.
Pediatric rate:
Manufacturer recommends 1 to 3 mg PE/kg/min for pediatric patients. Another source suggests a rate of 1.6 mg PE/kg/min.
Actions
A water-soluble prodrug of phenytoin. Converts to phenytoin, phosphate, and formaldehyde within 15 minutes of IV/IM administration. An anticonvulsant, chemically related to barbiturates. Selectively stabilizes seizure threshold and depresses seizure activity in the motor cortex. Modulation of voltage-dependent sodium channels of neurons thought to be the primary cellular mechanism responsible for anticonvulsant activity. Also exerts a depressant effect on the myocardium by selectively elevating the excitability threshold of the cell, reducing the cell’s response to stimuli. Peak levels of fosphenytoin are achieved by the end of an infusion, but conversion to therapeutic serum levels of phenytoin takes longer. The conversion half-life of fosphenytoin to phenytoin is approximately 15 minutes. Extensively bound to protein, fosphenytoin displaces phenytoin from protein binding sites and increases free phenytoin (dose and rate dependent). Phenytoin’s half-life is 12 to 28.9 hours. It is metabolized in the liver by hepatic cytochrome P450 enzymes (CYP2C9 and CYP2C19) and excreted in urine. Crosses the placental barrier. Secreted in breast milk.
Indications and uses
Treatment and control of generalized tonic-clonic status epilepticus. ■ Treatment or prophylaxis of seizures in neurosurgical patients. ■ Substitute for oral phenytoin when oral administration is not feasible or prompt increases in antiepileptic drug levels are needed. Cerebyx must not be given orally.
Contraindications
Hypersensitivity to fosphenytoin, any of its components, phenytoin, or other hydantoins. ■ Sinus bradycardia, sinoatrial block, second- and third-degree AV block, and Adams-Stokes syndrome. ■ Coadministration with delavirdine (Rescriptor).
Precautions
Doses of fosphenytoin are expressed in terms of milligrams of phenytoin equivalents (mg PE) to avoid the need to perform molecular weight–based adjustments when substituting fosphenytoin for phenytoin or vice versa; no adjustment required when substituting fosphenytoin or vice versa. Labeling of fosphenytoin has been updated by the manufacturer to reduce the incidence of dosing errors. To ensure accuracy, confirm actual dose and volume to be administered with a pharmacist or another RN. ■ Advantages of fosphenytoin over present phenytoin products include solubility in IV solutions, improved infusion site tolerance, more rapid rate of administration, and well-tolerated IM option. ■ IV route indicated in emergency situations (e.g., status epilepticus). May be given IM in nonemergency situations. ■ Oral phenytoin is preferred in nonemergency situations because of the risks of cardiac and local toxicity associated with IV fosphenytoin. Adverse cardiovascular reactions have occurred during and after infusions and have been reported with infusion rates above the recommended rate as well as with infusion rates at or below the recommended rate. ■ May cause severe cardiovascular reactions (e.g., hypotension, bradycardia, various degrees of AV block, QT interval prolongation) that have resulted in asystole, cardiac arrest, and death; use extreme caution in elderly or seriously ill patients and in patients with hypotension or severe myocardial insufficiency. Risk of hypotension and cardiac arrhythmias increased by higher IV doses and/or rapid administration. ■ Intended for short-term parenteral use (up to 5 days). ■ Transfer to oral phenytoin therapy as soon as feasible. ■ Abrupt withdrawal may cause increased seizure activity. Gradually reduce dose, discontinue, or substitute alternative antiepileptic agents. ■ Discontinue immediately for hypersensitivity reactions; with caution substitute a nonhydantoin anticonvulsant. ■ In patients who have experienced phenytoin hypersensitivity, alternatives to structurally similar drugs such as carboxamides (e.g., carbamazepine [Tegretol]), barbiturates, succinimides, and oxazolidinediones (e.g., trimethadione) should be considered. ■ May exacerbate porphyria. ■ Drug reaction with eosinophilia and systemic symptoms (DRESS), also known as multiorgan hypersensitivity, has been reported. DRESS usually presents with fever, rash, and/or lymphadenopathy in association with other organ system involvement such as hepatitis, nephritis, hematologic abnormalities, myocarditis, or myositis. Eosinophilia is often present. Deaths have been reported. ■ May cause acute phenytoin hepatotoxicity, which may manifest as elevated liver function tests, jaundice, hepatomegaly, leukocytosis, eosinophilia, and/or acute hepatic failure. These events may be part of the spectrum of DRESS or may occur in isolation. Discontinue immediately and substitute alternate anticonvulsant therapy. ■ Has caused lymphadenopathy and hemopoietic complications (e.g., agranulocytosis, granulocytopenia, leukopenia, thrombocytopenia, or pancytopenia with or without bone marrow suppression). Lymph node involvement may occur with or without S/S resembling DRESS. If lymphadenopathy occurs, substitute alternate anticonvulsant therapy. ■ Sensory disturbances, including severe burning, itching, and paresthesia, have been reported; more common with higher doses and/or rates. ■ Purple glove syndrome (edema, discoloration, and pain distal to the injection site) has occurred and may or may not be associated with extravasation. The syndrome may not develop for several days. ■ Serious dermatologic reactions have been reported, including toxic epidermal necrolysis (TEN) and Stevens-Johnson syndrome (SJS). Usually occurs within 28 days but may occur later. If a rash develops, the patient should be evaluated for S/S of DRESS. ■ Use caution with low serum albumin levels, and adjust dose as indicated. Phenytoin is highly bound to serum protein, and a reduced albumin causes an increase in free drug availability and may increase toxicity. ■ Not effective for absence seizures; combined therapy required if both conditions present. ■ Not indicated for seizures due to hypoglycemia or other metabolic causes. ■ Inhibits insulin release and may increase serum glucose; monitoring indicated in diabetics. ■ May lower serum folate levels. ■ Antiepileptic drugs (AEDs) increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior. Some psychotic symptoms and/or behavioral changes resolved without intervention. Others required dose reduction or discontinuation of the antiepileptic agent. ■ Confusional states referred to as delirium, psychosis, encephalopathy or, rarely, irreversible cerebellar dysfunction have been reported when plasma phenytoin concentrations are sustained above the optimum range. At the first sign of acute toxicity, determination of plasma phenytoin concentration is recommended.
Monitor:
Monitor ECG, BP, and respirations continuously during loading dose and for at least 10 to 20 minutes after infusion is complete. ■ Allow fosphenytoin time to convert to phenytoin; accurate serum levels are not available until 2 hours after the end of an IV infusion or 4 hours after IM injection. Narrow margin of error between therapeutic and toxic dose. Plasma levels above 10 mcg/mL usually control seizure activity. The acceptable range is 5 to 20 mcg/mL (unbound phenytoin concentration of 1 to 2 mcg/mL). Toxicity begins with nystagmus at levels exceeding 20 mcg/mL. ■ Monitoring of unbound phenytoin concentrations may be more useful in patients with renal or hepatic impairment or in patients with hypoalbuminemia. ■ Observe for rash and discontinue if one appears. If rash is mild, fosphenytoin may be resumed when the rash has completely disappeared. Discontinue fosphenytoin if the mild rash occurs again, if the initial rash is serious in nature (e.g., exfoliative, purpuric, bullous), or if Stevens-Johnson syndrome or toxic epidermal necrolysis is suspected. Do not resume; consider alternative therapy. ■ Phosphate is produced as a metabolite; monitor in patients who require phosphate restriction (e.g., renal impairment). ■ Monitor patients who are gravely ill, have impaired liver or renal function or hypoalbuminemia, or are elderly. May show early signs of toxicity. ■ Determine absolute patency of vein; avoid extravasation. Not as alkaline as phenytoin. ■ Observe patient closely for signs of CNS side effects; see Precautions.
Patient education:
Report burning, itching, numbness, pain, or rash. ■ Consider birth control options; nonhormonal birth control recommended. ■ May increase the risk of suicidal thoughts and behavior. Promptly report emergence or worsening of the S/S of depression, any unusual changes in mood or behavior, or thoughts about self-harm. ■ Women who are pregnant or who become pregnant should be encouraged to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry.
Maternal/child:
Category D: avoid pregnancy. Prenatal exposure to phenytoin may increase the risk of congenital malformations and other adverse developmental outcomes. Consider risk versus benefit. ■ An increase in seizure frequency may occur during pregnancy; if phenytoin is required, monitoring of plasma phenytoin levels may be helpful. ■ Newborns whose mothers received phenytoin during pregnancy may develop a life-threatening bleeding disorder that can be prevented by giving vitamin K to the mother before delivery and to the neonate after birth. ■ Discontinue breast-feeding. ■ Safety and effectiveness for use in pediatric patients not established. ■ See Patient Education.
Elderly:
See Dose Adjustments. ■ Sensitivity and/or toxicity may be increased because serum concentrations may be elevated due to reduced clearance, or low serum albumin may cause a decrease in protein binding and an increase in free phenytoin.
Drug/lab interactions
No drugs are known to interfere with the conversion of fosphenytoin to phenytoin, although phosphatase activity may have an impact. ■ Capable of innumerable catastrophic drug interactions; review of drug profile by pharmacist imperative. In all situations, monitoring of phenytoin serum levels may be indicated. ■ Coadministration with delavirdine (Rescriptor) is contraindicated. Has the potential for loss of virologic response and possible resistance to delavirdine or to the class of nonnucleoside reverse transcriptase inhibitors. ■ Serum levels and toxicity of phenytoin may be increased by alcohol (acute intake), amiodarone (Nexterone), antiepileptic agents (e.g., felbamate [Felbatol], topiramate [Topamax], oxcarbazepine [Trileptal]), azoles (e.g., fluconazole [Diflucan], itraconazole [Sporanox], ketoconazole (Nizoral), miconazole [Oravig], voriconazole [VFEND]), capecitabine (Xeloda), chloramphenicol, chlordiazepoxide (Librium), disulfiram (Antabuse), estrogens, fluorouracil, fluoxetine (Prozac), fluvastatin (Lescol), fluvoxamine (Luvox), H2 antagonists (e.g., cimetidine [Tagamet]), halothane, isoniazid (INH), methylphenidate (Ritalin), omeprazole (Prilosec), phenothiazines (e.g., prochlorperazine [Compazine]), salicylates (aspirin), sertraline (Zoloft), succinimides (e.g., ethosuximide [Zarontin]), sulfonamides (e.g., sulfamethizole, sulfaphenazole, sulfadiazine, sulfamethoxazole/trimethoprim [Bactrim]), ticlopidine (Ticlid), tolbutamide, trazodone (Deseryl), and warfarin (Coumadin). ■ Serum levels and effectiveness of phenytoin may be decreased by anticancer drugs usually in combination (e.g., bleomycin [Blenoxane], carboplatin [Paraplatin], cisplatin, doxorubicin [Adriamycin], methotrexate), carbamazepine (Tegretol), chronic alcohol abuse, diazepam (Valium), diazoxide (Proglycem), folic acid, fosamprenavir (Lexiva), nelfinavir (Viracept), reserpine, rifampin (Rifadin), ritonavir (Norvir), St. John’s wort, theophylline, and vigabatrin (Sabril). ■ Serum levels of phenytoin may be increased or decreased by phenobarbital (Luminal), valproate sodium (Depacon), valproic acid (Depakene). Similarly, phenytoin may unpredictably affect the levels and efficacy of these drugs. ■ Phenytoin will inhibit the effects of azoles (e.g., fluconazole [Diflucan], itraconazole [Sporanox], ketoconazole [Nizoral], posaconazole [Noxafil], voriconazole [VFEND]), corticosteroids, doxycycline, estrogens, furosemide (Lasix), irinotecan (Camptosar), oral contraceptives, paclitaxel (Taxol), paroxetine (Paxil), quinidine, rifampin (Rifadin), sertraline (Zoloft), teniposide (Vumon), theophylline, and vitamin D. Dose adjustment of these agents may be indicated. ■ Decreases plasma concentrations of active metabolites of albendazole (Albenza), antiepileptic agents (e.g., carbamazepine [Tegretol], felbamate [Felbatol], lamotrigine [Lamictal], oxcarbazepine [Trileptal], topiramate [Topamax]), atorvastatin (Lipitor), certain HIV antivirals (e.g., efavirenz [Sustiva], lopinavir/ritonavir [Kaletra], indinavir [Crixivan], nelfinavir [Viracept], ritonavir [Norvir], saquinavir [Invirase]), chlorpropamide (Diabinese), clozapine (Clozaril), cyclosporine (Sandimmune), digoxin (Lanoxin), fluvastatin (Lescol), folic acid, methadone (Dolophine), mexiletine, nifedipine (Procardia), nimodipine, nisoldipine (Sular), praziquantel (Biltricide), quetiapine [Seroquel], simvastatin (Zocor), and verapamil. Adjust doses of these agents as indicated. ■ May increase or decrease PT/INR responses when coadministered with warfarin (Coumadin). ■ When given with fosamprenavir (Lexiva) alone, phenytoin may decrease the concentration of amprenavir (Agenerase), the active metabolite. When given in combination with fosamprenavir and ritonavir (Norvir), phenytoin may increase the concentration of amprenavir. ■ Resistance to the neuromuscular blocking action of the nondepolarizing neuromuscular blocking agents pancuronium, vecuronium, rocuronium (Zemuron), and cisatracurium (Nimbex) has occurred in patients receiving long-term administration of phenytoin. Monitor for more rapid than expected recovery from neuromuscular blockade. ■ Alters some clinical laboratory tests (e.g., may decrease T4, increase glucose, alkaline phosphatase, and GGT; may produce low results in dexamethasone or metyrapone tests).
Side effects
Transient ataxia, dizziness, headache, nystagmus, paresthesia, pruritus, and somnolence are the most common side effects and are dose- and rate-related. Risks of side effects increased with upper-end doses given at upper-end rates. The more important adverse clinical events caused by IV use of fosphenytoin are cardiovascular collapse and/or CNS depression. Coma, hyperreflexia, hypotension, lethargy, nausea, slurred speech, tremor, and vomiting are also signs of increased toxicity. Confusional states (e.g., delirium, encephalopathy, psychosis) and, rarely, irreversible cerebellar dysfunction can occur with high plasma concentrations. Fosphenytoin breaks down into formate and phosphate metabolites that may cause formate and phosphate toxicity in overdose situations (hypocalcemia, metabolic acidosis, muscle spasms, paresthesia, and seizures). Psychotic symptoms, including aggression, agitation, anger, anxiety, apathy, depersonalization, depression, emotional lability, hallucinations, hostility, irritability, and suicidal tendencies, have occurred with antiepileptic agents.
Major/overdose:
Arrhythmias (e.g., asystole, bradycardia, cardiac arrest, tachycardia), ataxia, coma, dysarthria, hyperreflexia, hypocalcemia, hypotension, lethargy, metabolic acidosis, nausea, nystagmus, respiratory depression, slurred speech, syncope, tremor, and vomiting. Deaths have been reported.
Post-marketing:
Anaphylactoid reactions, including anaphylaxis.
Antidote
Notify physician of any side effects. Obtain serum plasma levels at first signs of toxicity; reduce dose. If symptoms persist or major side effects appear, discontinue fosphenytoin and notify physician. Treat symptomatically, maintain a patent airway, and resuscitate as necessary. Discontinue fosphenytoin at the first sign of a rash unless the rash is clearly not drug related. If TEN or SJS is suspected, do not rechallenge; consider alternate therapy. Evaluate for multiorgan hypersensitivity reactions (DRESS). Cardiovascular toxicity, including hypotension or cardiac arrhythmias, may respond to a decrease in infusion rate. One source says hemodialysis may be helpful in overdose. Another source says hemodialysis, peritoneal dialysis, forced fluid diuresis, exchange transfusions, and plasmapheresis are ineffective. In overdose, measure ionized free calcium levels to guide treatment in phosphate toxicity.
Furosemide 
(fur-OH-seh-myd)
Lasix
Diuretic (loop)
pH 8 to 9.3
Usual dose
Adjust dose and dose schedule to individual patient needs. Reserve parenteral therapy for emergent situations or for patients unable to take oral therapy. Switch to oral therapy as soon as practical.
Edema:
20 to 40 mg. May be repeated in 2 hours. If necessary, increase dosage by 20-mg increments (under close medical supervision and no sooner than 2 hours after previous dose) until desired diuresis is obtained. After the initial diuresis the minimum effective dose may be given once or twice every 24 hours as required for maintenance.
New-onset pulmonary edema:
40 mg. Dose may be increased to 80 mg after 1 hour if satisfactory response is not obtained. AHA guidelines recommend less than 0.5 mg/kg. Additional therapy (e.g., digoxin, oxygen) may be administered concurrently as needed.
Hypertensive crisis:
AHA guidelines recommend 0.5 to 1 mg/kg over 1 to 2 minutes. If no response, increase dose to 2 mg/kg.
Acute pulmonary edema or post–cardiac arrest cerebral edema:
AHA guidelines recommend 0.5 to 1 mg/kg over 1 to 2 minutes. If no response, increase dose to 2 mg/kg.
Pediatric dose
See Maternal/Child.
Diuretic:
1 to 2 mg/kg of body weight. After 2 hours increase by 1-mg/kg increments to effect desired response. Effective dose may be given every 6 to 12 hours. Another source suggests 1 to 2 mg/kg/dose every 6 to 12 hours. Do not exceed 6 mg/kg/dose.
Neonatal dose
Diuretic:
0.5 to 1 mg/kg/dose every 8 to 24 hours. Maximum dose is 2 mg/kg/dose. Literature reports suggest that the maximum dose for premature infants should not exceed 1 mg/kg/day. See Maternal/Child.
Dose adjustments
Higher doses may be required in renal insufficiency and acute or chronic renal failure. ■ Reduced dose or extended intervals may be appropriate in the elderly. ■ Extend dosing intervals in neonates because half-life is prolonged. ■ See Drug/Lab Interactions.
Dilution
May be given undiluted. May be given through Y-tube or three-way stopcock of infusion set. Not usually added to IV solutions, but large doses may be added to NS, LR, D5W, D5NS and given as an infusion. pH of solution must be over 5.5. Some sources recommend protecting diluted solutions from light to prevent photodegradation (minimized at pH 7).
Filters:
One source found no significant drug loss when filtered through a 0.22-micron filter.
Storage:
Store vials at CRT. Protect from light. If diluted for infusion, discard after 24 hours.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Furosemide may precipitate at a pH below 7. Manufacturer states, “Acid solutions, including other parenteral medications (e.g., amrinone, ciprofloxacin, labetalol, milrinone) must not be administered concurrently in the same infusion.” Additionally, “Furosemide should not be added to an IV line containing any of these acidic products.”
One source suggests the following compatibilities:
Additive:
Amikacin, aminophylline, amiodarone (Nexterone), ampicillin, atropine, bumetanide, calcium gluconate, cefuroxime (Zinacef), dexamethasone (Decadron), digoxin (Lanoxin), epinephrine (Adrenalin), gentamicin, heparin, hydrocortisone sodium succinate (Solu-Cortef), lidocaine, mannitol (Osmitrol), meropenem (Merrem IV), midazolam (Versed), morphine, nitroglycerin IV, potassium chloride (KCl), ranitidine (Zantac), sodium bicarbonate, theophylline, tobramycin, verapamil.
Y-site:
Allopurinol (Aloprim), amifostine (Ethyol), amikacin, amiodarone (Nexterone), amphotericin B cholesteryl (Amphotec), anidulafungin (Eraxis), argatroban, aztreonam (Azactam), bivalirudin (Angiomax), bleomycin (Blenoxane), cefepime (Maxipime), ceftaroline (Teflaro), ceftazidime (Fortaz), cisatracurium (Nimbex), cisplatin, cladribine (Leustatin), cyclophosphamide (Cytoxan), dexmedetomidine (Precedex), dobutamine, docetaxel (Taxotere), dopamine, doripenem (Doribax), doxorubicin liposomal (Doxil), epinephrine (Adrenalin), etoposide phosphate (Etopophos), famotidine (Pepcid IV), fentanyl, fludarabine (Fludara), fluorouracil (5-FU), foscarnet (Foscavir), gallium nitrate (Ganite), granisetron (Kytril), heparin, hetastarch in electrolytes (Hextend), hydrocortisone sodium succinate (Solu-Cortef), hydromorphone (Dilaudid), 6% hydroxyethyl starch (Voluven), indomethacin (Indocin IV), leucovorin calcium, linezolid (Zyvox), lorazepam (Ativan), melphalan (Alkeran), meperidine (Demerol), meropenem (Merrem IV), methotrexate, metoprolol (Lopressor), micafungin (Mycamine), mitomycin (Mutamycin), morphine, nitroglycerin IV, nitroprusside sodium, norepinephrine (Levophed), oxaliplatin (Eloxatin), paclitaxel (Taxol), pantoprazole (Protonix IV), piperacillin/tazobactam (Zosyn), potassium chloride (KCl), propofol (Diprivan), ranitidine (Zantac), remifentanil (Ultiva), sargramostim (Leukine), tacrolimus (Prograf), teniposide (Vumon), thiotepa, tirofiban (Aggrastat), tobramycin.
Rate of administration
IV injection:
Each 40 mg or fraction thereof should be given over 1 to 2 minutes.
Infusion:
Adults:
0.1 mg/kg/hr.
Pediatric patients:
0.05 mg/kg/hr. Titrate to effect. High-dose therapy in an infusion should not exceed a rate of 4 mg/min.
Actions
A potent loop diuretic, structurally related to sulfonamides. Onset of action is prompt, usually within 5 minutes. Duration of action and half-life are approximately 2 hours. Inhibits the reabsorption of sodium and chloride in the proximal and distal tubules and in the loop of Henle, causing increased excretion of water, sodium, chloride, magnesium, and calcium. Highly protein bound. Metabolized and excreted in the urine. Crosses the placental barrier. Secreted in breast milk.
Indications and uses
Parenteral therapy should be reserved for patients unable to take oral medications or for emergency clinical situations. ■ Edema associated with congestive heart failure, cirrhosis of the liver with ascites, and renal disease including the nephrotic syndrome. Particularly useful when an agent with a greater diuretic potential is required. ■ Adjunctive therapy in acute pulmonary edema. Indicated when rapid onset of diuresis is necessary (e.g., acute pulmonary edema).
Contraindications
Anuria, hypersensitivity to furosemide.
Precautions
A potent diuretic; may precipitate excessive diuresis with water and electrolyte depletion. Careful medical supervision is required. ■ Use caution and improve basic condition first in hepatic coma, electrolyte depletion, and advanced cirrhosis of the liver. ■ May be used concurrently with aldosterone antagonists (e.g., spironolactone [Aldactone]) for more effective diuresis and to prevent excessive potassium loss. ■ If increasing azotemia and oliguria develop during treatment of severe progressive renal disease, furosemide should be discontinued. ■ Use extreme caution in known sulfonamide sensitivity. ■ Risk of ototoxicity (tinnitus, reversible or irreversible hearing impairment and deafness) increases with higher doses, rapid injection, severe renal dysfunction, hypoproteinemia, or concurrent use with other ototoxic drugs; see Drug/Lab Interactions. ■ May activate or exacerbate systemic lupus erythematosus. ■ Use caution in patients with severe symptoms of urinary retention (because of bladder emptying disorders, prostatic hypertrophy, urethral narrowing). May experience acute urinary retention related to increased production and retention of urine.
Monitor:
Monitor BP frequently, especially during initial therapy. ■ May precipitate excessive diuresis with water and electrolyte depletion. Routine checks on electrolyte panel, CO2, and BUN are necessary during therapy. Electrolyte replacement may be required. ■ May increase blood glucose and has precipitated diabetes mellitus. ■ Monitor for other S/S of fluid or electrolyte imbalance, which may include dryness of the mouth, drowsiness, lethargy, muscle cramps or fatigue, nausea or vomiting, oliguria, tachycardia, thirst, and weakness. ■ May lower serum calcium level; may cause tetany. ■ Hyperuricemia can occur. Rarely precipitates acute gout attack. ■ See Drug/Lab Interactions.
Patient education:
Hypotension may cause dizziness; request assistance with ambulation. ■ Report cramps, dizziness, muscle weakness, or nausea promptly. ■ May cause a decrease in potassium levels and require a supplement. ■ Skin may become photosensitive; avoid unprotected exposure to sun. ■ Therapy for diabetes may require adjustment; monitoring of serum glucose required.
Maternal/child:
Category C: use during pregnancy only when clearly needed and benefits outweigh potential risks to fetus. Treatment during pregnancy requires monitoring of fetal growth because of the potential for higher fetal birth weights. ■ Use caution in breast-feeding; may inhibit lactation. ■ Safety for use in pediatric patients not established. ■ Prolonged use in premature infants may result in nephrocalcinosis and nephrolithiasis. Has also been observed in pediatric patients under 4 years of age who have been treated chronically with furosemide. ■ May increase risk of persistent patent ductus arteriosus in preterm infants with respiratory distress syndrome. ■ Premature infants receiving doses exceeding 1 mg/kg/day may develop plasma levels that could be associated with toxicity, including ototoxicity. Hearing loss in neonates has been associated with the use of furosemide.
Elderly:
Protein binding and clearance is decreased in the elderly. ■ Consider increased sensitivity to hypotensive and electrolyte effects. Dose selection should be cautious, starting at the lower end of the dosing range. ■ May be more susceptible to dehydration; observe carefully. ■ Avoid rapid contraction of plasma volume and hemoconcentration. May cause thromboembolic episodes (e.g., CVA, pulmonary emboli). ■ The initial diuretic effect in older patients is decreased relative to younger patients.
Drug/lab interactions
Causes excessive potassium depletion with corticosteroids, thiazide diuretics (e.g., hydrochlorothiazide), amphotericin B (all formulations). ■ Potentiates antihypertensive drugs (e.g., angiotensin-converting enzyme [ACE] inhibitors [e.g., enalapril (Vasotec), enalaprilat (Vasotec IV), lisinopril (Zestril)], angiotensin II receptor blockers [e.g., losartan (Cozaar)], nitroglycerin, nitroprusside sodium); reduced dose of the antihypertensive agent or both drugs may be indicated. ■ May cause transient or permanent deafness with doses exceeding the usual or when given in conjunction with other ototoxic drugs (e.g., aminoglycosides [e.g., gentamicin], cisplatin). ■ Amphotericin B (all formulations) may increase potential for ototoxicity and nephrotoxicity; avoid concurrent use. ■ Nephrotoxicity increased by other nephrotoxic agents (e.g., aminoglycosides, cephalosporins [e.g., ciprofloxacin (Cipro)], cisplatin, cyclosporine [Sandimmune], radiocontrast agents, vancomycin); avoid concurrent use. ■ May increase serum levels of lithium (may cause toxicity). ■ May cause cardiac arrhythmias with amiodarone (Nexterone) or digoxin (potassium depletion). ■ May enhance or inhibit actions of nondepolarizing muscle relaxants (e.g., atracurium [Tracrium]). ■ May potentiate the action of succinylcholine. ■ Effects may be inhibited by NSAIDs (e.g., ibuprofen [Motrin]) or probenecid. ■ May cause profound diuresis and serious electrolyte abnormalities with thiazide diuretics (e.g., chlorothiazide [Diuril]) because of synergistic effects. ■ May be inhibited by phenytoin (Dilantin). ■ Do not use concomitantly with ethacrynic acid (Edecrin); risk of ototoxicity markedly increased. ■ Salicylates may decrease the diuretic effect of furosemide. ■ Furosemide may increase serum levels of salicylates, increasing the risk of toxicity. ■ May decrease arterial responsiveness to norepinephrine. Dose adjustment may be required. ■ May enhance adverse effects of chloral hydrate. Concomitant use not recommended. ■ Methotrexate and other drugs that undergo significant renal tubular secretion may decrease the effectiveness of furosemide. ■ Furosemide may decrease renal elimination of other drugs that undergo tubular secretion. High-dose treatment of both furosemide and these other drugs may result in elevated serum levels and may potentiate toxicity. ■ Concomitant use of cyclosporine and furosemide has been associated with increased risk of gouty arthritis secondary to furosemide-induced hyperuricemia and cyclosporine impairment of renal urate excretion. ■ See Precautions.
Side effects
Usually occur in prolonged therapy, seriously ill patients, or following large doses.
Anemia, anorexia, blurring of vision, deafness (reversible), diarrhea, dizziness, elevated hepatic enzymes, eosinophilia, headache, hyperglycemia, hyperuricemia, hypokalemia, increases in serum cholesterol and triglyceride levels, leg cramps, lethargy, leukopenia, mental confusion, nausea, paresthesia, postural hypotension, pruritus, rash, tinnitus, urinary frequency, urticaria, vomiting, weakness.
Major:
Anaphylactic shock, blood volume reduction, circulatory collapse, dehydration, excessive diuresis, hepatic encephalopathy in patients with hepatic insufficiency, hypokalemia, metabolic acidosis, Stevens-Johnson syndrome, toxic epidermal necrolysis, vascular thrombosis and embolism.
Overdose:
Blood volume reduction, dehydration, electrolyte imbalances, hypochloremic alkalosis, and hypotension.
Antidote
If minor side effects are noted, discontinue the drug and notify the physician, who may treat the side effects symptomatically and continue the drug. If side effects are progressive or any major side effect occurs, discontinue the drug immediately and notify the physician. Treatment of major side effects is symptomatic and aggressive. Monitor serum electrolytes, carbon dioxide level, and BP frequently, and replace excessive fluid and electrolyte losses as needed. Hemodialysis does not accelerate furosemide elimination. Resuscitate as necessary.

Full access? Get Clinical Tree


