Chapter 25 Female reproductive physiology
After reading this chapter, you will be able to:
Introduction
From puberty to menopause, ovarian activity is characterized by cyclic development of several follicles; maturation and ovulation of a selected follicle, and subsequent formation and regression of the corpus luteum. This episodic pattern of ovulation requires cyclical release of steroid and peptide hormones that imposes a corresponding cyclicity on many organ systems; levels of sexual arousability; and differing behaviours (Chapman et al 1998, Chidambaram et al 2002, Haselton et al 2007, Salonia et al 2005, Tarin et al 2002, Webb 1986).
The ovarian cycle
The ovarian cycle has three distinct phases:
In humans, the first and third phases take approximately 14 days each, while the second takes about 15 minutes (Lousse & Donnez 2008) (Fig. 25.1).
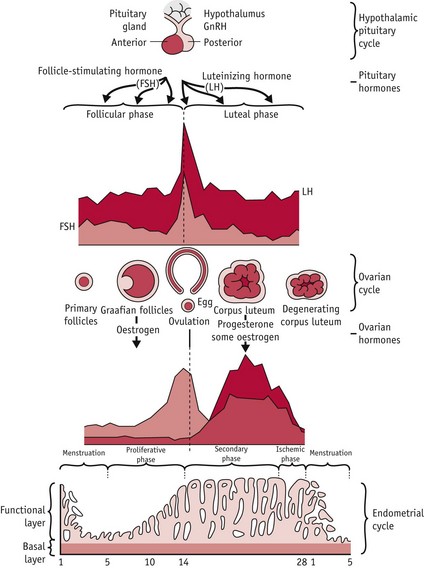
Figure 25.1 Hypothalamic–pituitary–ovarian–endometrial changes during an ovulatory cycle.
(Reproduced with permission from Blackburn 2007:36.)
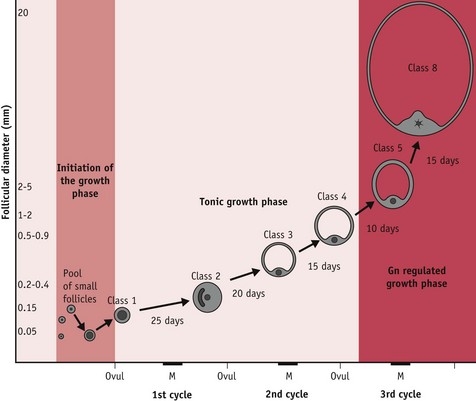
Of the 15–20 follicles recruited, one selected follicle demonstrates increased cell proliferation and differentiation; expansion of thecal vascularity; rapid accumulation of antral fluid; and rising capacity for steroid and peptide hormone secretion (Geva & Jaffe 2000, Gougeon 1996, Seifer et al 2002). Following selection, this growing follicle regulates changes in hypothalamic–pituitary gonadotrophin activity during oocyte maturation and ovulation, and following the mid-cycle LH/FSH surge, when it differentiates into a functional corpus luteum or postovulatory follicle (Baker & Spears 1999, Hodgen 1982, Kunz et al 1998, Nakao et al 2007) (Fig. 25.2).
At mid-cycle, the dominant follicle becomes visible to the naked eye as a large bulge under the surface epithelium of the ovary. The enclosed oocyte acquires meitotic and developmental competence and subsequently undergoes a profound reorganization of the nucleus and cytoplasm, in preparation for fertilization and blastocyst formation (Canipari 2000, Gilchrist et al 2008).
Ovarian regulation of gonadotrophins
The ovary contains thousands of highly differentiated cells and functions as a complex neuroendocrine–autocrine–paracrine gland, synchronizing hypothalamic, pituitary, ovarian, uterine and mammary activities, throughout the cycle (Behrens et al 1995, Hirshfield 1991, Kunz et al 1998, Nakao et al 2007, Armstrong & Webb 1997).
The ovarian cycle temporarily modulates the secretory patterns of pituitary and hypothalamic gonadotrophins, through a changing balance of hormonal secretions, in successive cohorts of antral follicles, the dominant follicle and its successor, the postovulatory follicle or corpus luteum (Baker & Spears 1999, Hodgen 1982). Across the cycle, ovarian steroid and peptide hormones act through negative and positive feedback mechanisms on the synthesis and pattern of release of hypothalamic gonadotrophin-releasing hormone (GnRH); pituitary gonadotrophins FSH and LH and their responsiveness to GnRH, in conjunction with other modulatory hormones and neurotransmitters operating on pituitary and hypothalamic gonadotrophin cells (Ball 2007, Nakao et al 2007, Smith & Jennes 2001).
Antral fluid has a key role in follicular growth, oocyte maturation, ovulation and fertilization (Tse et al 2002).
As the dominant follicle increases in size (Fig. 25.3), rising synthesis of oestrogens and progesterone lead to a parallel rise in circulating concentrations.
Mid-cycle LH/FSH surge
The LH/FSH surge is temporally associated with attainment of peak secretion of oestrogens following a rapid rise in progesterone 12 hours earlier (Micevych & Sinchak 2008, Stouffer 2003). In the hypothalamus, oestrogens induce a rise in synthesis of neuroprogesterone and neuroprogesterone receptors, and these events initiate the pulsatile release of GnRH, which then stimulates the surge release of LH needed for ovulation (Micevych & Sinchak 2008, Smith & Jennes 2001) (Fig. 25.4).
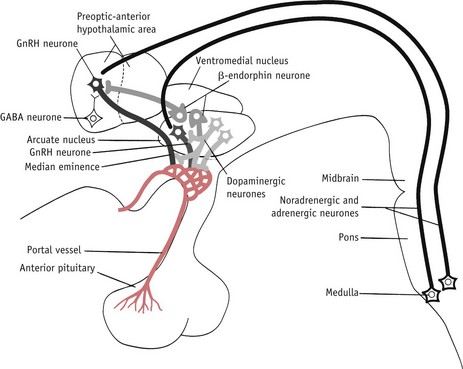
Figure 25.4 Some of the postulated neurochemical reactions that may control GnRH secretion.
(Reproduced with permission from Johnson 2007:116.)
During the ovulatory cycle, oxytocin levels vary in the hypothalamo-hypophysial complex, medulla and cerebrospinal fluid (Evans 1996, Salonia et al 2005). In the peripheral circulation, higher levels of oxytocin coincide with the mid-cycle LH surge – variations have been found in oxytocin and oxytocin receptors in granulosa and stromal cells, as well as in uterine and cervical tissue across the ovarian cycle (Behrens et al 1995, Kunz et al 1998). Evidence suggests that central oxytocin plays a key role in regulating the LH surge, by acting directly on GnRH neurons and stimulating sexual behaviour around ovulation.
Uterine and cervical oxytocin cooperate in conjunction with prostaglandin E2 (PGE2)and the preovulatory surge in oestrogens from the dominant follicle. These interactions promote fertilization, by softening cervical tissue and stimulating cervico-fundal contractions, to rapidly transport waves of spermatozoa from the posterior vaginal fornix to the isthmic portion of the fallopian tube (Behrens et al 1995, Drobnis & Overstreet 1992, Kunz et al 1998).
The preovulatory follicle
Following the LH/FSH surge, fibroblasts within the theca externa lay down connective tissue and theca interna and mural granulosa cells begin to differentiate from a predominantly oestrogen-secreting tissue into a highly vascularized corpus luteum with progesterone as its major steroid hormone and a rapid rise in progesterone receptors (Stouffer 2003).
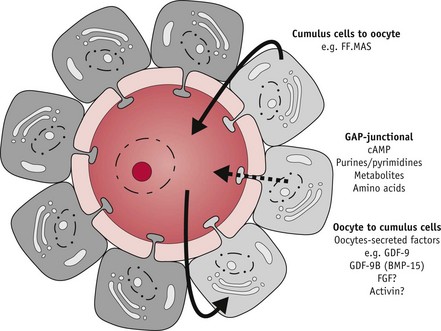
During the critical preovulatory period, the oocyte also secretes molecules that enhance oestrogens and inhibit progesterone secretion by cumulus granulosa cells (Gilchrist et al 2008, Sutton et al 2003) which also express oxytocin receptors. Oxytocin appears to modulate progesterone secretion while stimulating progesterone receptors on granulosa cells and a variety of regulatory molecules, including growth factors, integrins, prostaglandins and intracellular messengers involved in the process of ovulation.
Oocyte maturation
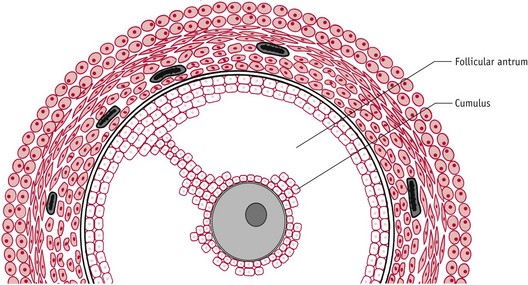
Throughout the growth phase of the oocyte, progression of meiosis is arrested at the diplotene or germinal vesicle stage of prophase 1. Within 12 hours of the LH/FSH surge, the fully grown oocyte is reactivated to briefly resume meiosis, while complex maturational changes occur in the cytoplasm to support fetilization and the surrounding cumulus cells expand (Sutton-McDowall et al 2010). FSH receptors are present on the entire surface of human oocytes, suggesting that FSH has direct control of oocyte maturation (Meduri et al 2002).
Preparation for the corpus luteum
Prior to ovulation, mural granulosa and theca interna cells differentiate predominantly into progesterone-secreting theca-lutein and granulosa-lutein cells that remain in the ovary following ovulation, and rapidly expand to form the highly vascularized corpus luteum (Niswender et al 2000, Stouffer 2003) (see website).
Ovulation
The LH/FSH surge dramatically increases blood flow and vascular permeability in thecal capillary networks that descend to the basal lamina. The rapid increase in size and vascularization of the follicle is accompanied by the local release of the prostaglandin PGE2 and of vasodilatory substances like histamine and bradykinin. PGE2 initiates the breakdown of collagen fibres within the thecal compartment, and other molecules cause an inflammatory reaction from within. FSH and progesterone also initiate proteolytic enzyme activity that loosens, distends and finally erodes the follicle wall at its weakest point (Brannstrom et al 1996).
Between 36 and 42 hours following the surges, the apex of the follicle begins to bulge below the surface of the ovary, rupturing at its weakest point, allowing antral fluid to flow into the peritoneal cavity, carrying the expanded oocyte–cumulus complex into the fallopian tube (Brannstrom et al 1996).
At ovulation, the dominant follicle releases the hugely expanded oocyte–cumulus complex from the surface of the ovary (Fig. 25.5). Immediately afterwards, the cumulus cell mass regulates acceptance of the oocyte by the fimbriae of the fallopian tube and subsequently functions as a hormonal and metabolic unit in the lumen of the uterine cavity, optimizing conditions for the enclosed oocyte and zona pellucida to undergo final maturational changes in preparation for fertilization, implantation, and embryo formation (Talbot et al 2003).