Psychopharmacology
In contemporary [psychiatric] treatment, psychological and psychopharmacologic models are highly compatible. When used in a combination or matrix model, the clinical outcomes are positive and powerful in enhancing quality of life for both the client and family and improving functional status.
The use of drugs to treat psychiatric disorders is often the foundation for a successful treatment approach that can also include other types of interventions such as psychotherapy or behavioral therapies.
Learning objectives
After studying this chapter, you should be able to:
Articulate how the terms pharmacodynamics and pharmacokinetics relate to the science of psychopharmacology.
Differentiate primary, secondary, and tertiary effects of psychotropic drugs.
Identify implications of drug polymorphism, discontinuation syndrome, neuroleptic malignant syndrome, serotonin syndrome and metabolic syndrome in the psychiatric setting.
Explain the rationale for the administration of each of the following: antipsychotic agents/neuroleptics, antianxiety agents and hypnotics, antidepressants, stimulants used as mood elevators, antimanic agents used as mood stabilizers, anticonvulsants used as mood and behavior stabilizers, and antiparkinsonism or anticholinergic agents to treat medication-induced movement disorders.
Recognize the contraindications for and possible adverse effects of the following: antipsychotic agents/neuroleptics, antianxiety agents and hypnotics, antidepressants, stimulants, antimanic agents, anticonvulsants, and antiparkinsonism or anticholinergic agents.
Explain the nursing implications when administering various classifications of psychotropic drugs.
Demonstrate an understanding of the importance of client and family education regarding psychotropic drugs.
Key Terms
Acute dystonia
Acute dyskinesia
Akathisia
Atypical antipsychotics
Clearance
Clinical efficacy
Clinical psychopharmacology
Conventional antipsychotics
Discontinuation (withdrawal) syndrome
Drug half-life
Drug polymorphism
Extrapyramidal adverse effects (EPS)
First pass effects
Median effective dose
Median toxic dose
Metabolic syndrome
Neuroleptic malignant syndrome (NMS)
Neuroleptics
Parkinsonism
Peak plasma concentration
Pharmacodynamics
Pharmacokinetics
Potency
Primary effects
Psychopharmacology
Secondary effects
Serotonin syndrome
Tardive dyskinesia (TD)
Tertiary effects
Therapeutic index
Therapeutic window
Tolerance
Typical antipsychotics
Psychopharmacology is the study of the regulation and stabilization of emotions, behavior, and cognition through the interactions of endogenous signaling substances or chemicals in the brain, such as acetylcholine, dopamine, glutamate, norepinephrine, or serotonin, with drugs (Wilcox & Gonzales, 1998). Clinical psychopharmacology is the study of drug effects in clients and the expert use of drugs in the treatment of psychiatric conditions. Abnormalities in emotions, behavior, and cognition are assumed to be caused by biochemical alterations of neurotransmitters and their functions in the brain. Clinical symptoms are generally lessened when the biochemical alterations are corrected by pharmacotherapy.
The introduction of new psychotropic agents is perhaps the most rapidly growing area in the fields of psychiatry and clinical pharmacology. In recent years, important new drugs have been introduced, revolutionizing the treatment of various psychiatric disorders. This progress has been made possible through advances in neuroscience and clinical research during the last few decades (Puzantian & Stimmel, 2001).
This chapter focuses on the following classifications of psychotropic agents, also referred to as psychoactive or psychotherapeutic drugs:
Antipsychotic agents/neuroleptics
Antianxiety agents and hypnotics
Antidepressants
Stimulants used as mood elevators
Antimanic agents used as mood stabilizers
Anticonvulsants used as mood and behavior stabilizers
Antiparkinsonism or anticholinergic agents that are used to alleviate extrapyramidal symptoms or adverse effects of psychotropic agents
Each classification is discussed with the focus on principles or rationale for therapy; contraindications, precautions, and adverse effects; implications for nursing actions; and client and family education when applicable. Med Alert boxes are included, focusing on drug–drug interactions and unusual adverse effects. Daily dosage ranges for each classification of drugs, listed by both generic and trade name, are also given. Psychotropic agents used to treat specific disorders such as dementia, eating disorders, or substance abuse are discussed in the appropriate chapters. Finally, the back-of-book CD-ROM lists drugs used in the treatment of psychiatric and neurologic disorders.
The Science of Psychopharmacology
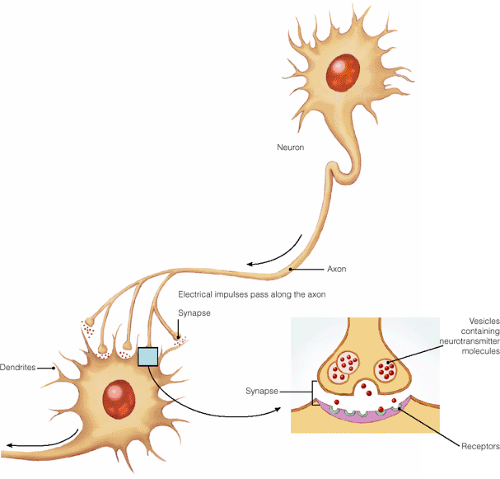
The science of psychopharmacology focuses on neurotransmission, the sending of impulses from one neuron to another across the synapse (the region surrounding the point of contact between two neurons or between a neuron and an effector organ) via the aid of specific substances called neurotransmitters. The last few decades have seen an explosion in the amount of data concerning the molecular biology of neurotransmission.
Neurotransmitters—chemicals such as acetylcholine, dopamine, glutamate, norepinephrine, and serotonin—reside in tiny sacs at the end of axons, the long tube-like parts of neurons, and are released when electrical impulses pass along the axon. When neurotransmitters cross the synapse, they undergo six steps in synaptic transmission as they bind to receptors on the surface of the next neuron (postsynaptic response; see Figure 16-1 for how neurons communicate). These steps include synthesis, vesicular uptake, transmitter release, receptor binding, cellular uptake, and transmitter metabolism. Each of these steps offers a potential target for therapeutic intervention by a drug. For example, certain drugs
can enhance the release of neurotransmitters, block neurotransmitter synthesis, or inhibit the cellular uptake of neurotransmitters (Wilcox & Gonzales, 1998).
can enhance the release of neurotransmitters, block neurotransmitter synthesis, or inhibit the cellular uptake of neurotransmitters (Wilcox & Gonzales, 1998).
As more groups and subgroups of neurotransmitter receptors are isolated and categorized, our understanding of psychopharmacology has broadened and has become useful in predicting future lines of research.
The interconnectedness of various neural subsystems is taken into account when attempting to explain pharmacologic data and the pathophysiology of mental illness. For example, typical antipsychotic drugs such as haloperidol (Haldol) block post-synaptic dopamine receptors; atypical antipsychotic drugs such as risperidone (Risperdal) block both dopamine and serotonin receptors; antidepressant drugs such as sertraline (Zoloft) or paroxetine (Paxil) (selective serotonin reuptake inhibitors, or SSRIs) bind with the serotonin transporter and inhibit reuptake of serotonin into the presynaptic neurons; and anxiolytic drugs such as lorazepam (Ativan) enhance gamma-aminobutyric acid (GABA) neurotransmission (Khan, 1999; Peterson, 2001; Sadock & Sadock, 2003).
The use of psychotropic drugs leads to changes in emotions, behavior, and cognition caused by the drug’s
primary, secondary, or tertiary effects. Primary effects occur as a drug is synthesized, released, and metabolized, and as it acts on the receptor sites of a neurotransmitter system (ie, noradrenaline, acetylcholine, dopamine, serotonin, glutamate, or GABA). Secondary effects result from interactions among the neurotransmitters, neuropeptides, and hormones as they influence each other’s function in the brain. For example, dopamine exerts a tonic inhibitory effect on the release of acetylcholine. Tertiary effects are the final changes in the clinical symptoms induced by a drug, such as the stabilization of anxiety or depression (Khan, 1999).
primary, secondary, or tertiary effects. Primary effects occur as a drug is synthesized, released, and metabolized, and as it acts on the receptor sites of a neurotransmitter system (ie, noradrenaline, acetylcholine, dopamine, serotonin, glutamate, or GABA). Secondary effects result from interactions among the neurotransmitters, neuropeptides, and hormones as they influence each other’s function in the brain. For example, dopamine exerts a tonic inhibitory effect on the release of acetylcholine. Tertiary effects are the final changes in the clinical symptoms induced by a drug, such as the stabilization of anxiety or depression (Khan, 1999).
Two major aspects of pharmacology, including psychopharmacology, are pharmacodynamics and pharmacokinetics.
Pharmacodynamics
Pharmacodynamics is the study of the biochemical and physiologic effects of drugs and the mechanisms by which the effects are produced. Put simply, pharmacodynamics refers to the effects of a drug on the body (TheFreeDictionary.Com, 2005a). Receptors for a drug are cellular components to which a drug binds and through which it initiates its pharmacodynamic effects. The drug can be an agonist for a receptor and stimulate the specific biologic activity of the receptor or an antagonist that inhibits biologic activity (Sadock & Sadock, 2003). Several terms are used to describe the effects of a drug on the body. They include:
Potency of a drug refers to the relative dosage of a drug that is required to achieve a desired effect. For example, 10 mg of the antidepressant paroxetine (Paxil) would be considered as potent as 100 mg of the antidepressant sertraline (Zoloft) if it achieved the same effect as sertraline.
Clinical efficacy refers to the maximum clinical response achievable by the administration of a specific drug. For example, a client whose clinical symptoms are stabilized by 10 mg of paroxetine (Paxil) exhibits maximum clinical efficacy, often reported as a percentage such as 90% or 100%.
Median effective dose is the dosage at which 50% of clients experience a specific therapeutic effect when prescribed a certain psychotropic drug. Blood levels below the median effective dose are associated with a poor response to the specific drug.
Median toxic dose is the dosage at which 50% of clients experience a specific toxic effect when taking a prescribed drug. Blood levels above the median dose may precipitate a toxic state.
Therapeutic index or therapeutic window has been defined as the ratio of the median effective dose to the median toxic dose.
Tolerance refers to the need for markedly increased amounts of a specific drug over time to achieve the same desired effect (Sadock & Sadock, 2003; Shahrokh & Hales, 2003).
The psychiatric–mental health nurse must be familiar with these terms because they are used in a variety of clinical settings such as substance abuse units; during clinical research studies involving psychotropic agents; and when agents are prescribed for special populations, such as older adults and children.
Pharmacokinetics
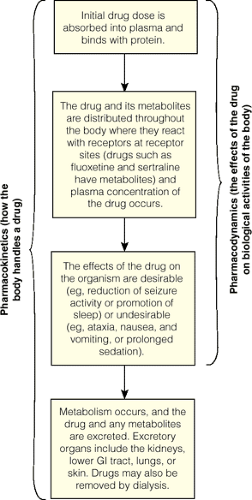
Pharmacokinetics is the study of the movement of drugs and their metabolites through the body by the process of drug absorption, distribution, metabolism, and excretion or elimination (TheFree-Dictionary.Com, 2005b). Pharmacokinetics, put simply, refers to how the body handles the drug. After the drug is ingested, it is absorbed into the bloodstream and distributed to various parts of the body in the form of a free or protein-bound drug. Only the free fraction can pass through the blood–brain barrier. The distribution of a drug to the brain is governed by the brain’s regional blood blow, the blood–brain barrier, and the drug’s affinity for its receptors in the brain. High cerebral blood flow, high lipid solubility, and high receptor affinity promote the therapeutic actions of the drug (Sadock & Sadock, 2003). The unbound protein of the drug is then transported to the liver, the principal site of metabolism, where it is changed into a more readily excreted form that is subsequently excreted from the body (eg, bile, feces, urine). Psychotropic drugs are also excreted in sweat, tears, and breast milk (Sadock & Sadock, 2003).
Most psychotropic drugs are metabolized by the cytochrome P-450 (CYP) hepatic enzyme system, which contains more than 30 isoenzymes referred to as metabolizing subsystems (eg, CYP1A2, CYP2D6, CYP2C9, CYP3A3). The phase 1 enzymatic process of metabolism involves oxidation. During this phase, a specific drug is reduced to form a more water-soluble compound. Smoking, environment, the ethnic origin or age of the client, or pollutants all can influence phase 1 metabolism. The effects of the influence of these factors are unpredictable because they may increase or decrease drug metabolism. During the phase 2 enzymatic process, a compound (such as glucuronide) is added to the parent drug or the phase
1–metabolized drug to enhance water solubility and promote excretion by the kidney or gastrointestinal (GI) tract (Deitch, 2003).
1–metabolized drug to enhance water solubility and promote excretion by the kidney or gastrointestinal (GI) tract (Deitch, 2003).
The addition of other drugs may increase or decrease psychotropic drug metabolism. For example, drugs may be identified as substrates, inducers, or inhibitors. Substrates are drugs metabolized by an isoenzyme in the CYP hepatic enzyme system. Amitriptyline (Elavil) is an example of a substrate that is metabolized in several of the subsystems. Substrates can be affected by an inducer or inhibitor. Inducers are drugs that, when administered at the same time as a substrate in the same metabolic system in the liver, are likely to speed up the metabolism of a substrate. For example, phenobarbital (Barbita) and carbamazepine (Tegretol) are inducers that would speed up the metabolism of the substrate amitriptyline (Elavil). Therefore, amitriptyline is metabolized quicker in the body and eliminated before it has a chance to be most effective. The effects of enzyme induction are sometimes more difficult to predict because these are dependent on drug half-lives, the rate of enzyme production, and individual genetic variations. Inhibitors are drugs that compete with another drug such as a substrate for enzyme binding sites. They block the process of metabolism of another drug when given concurrently and metabolized by the same metabolic system in the liver. For example, fluvoxamine (Luvox) and paroxetine (Paxil) are psychotropic inhibitors that slow or block the metabolism of amitriptyline (Elavil). Therefore, toxicity or toxic adverse effects can occur as a result of an elevation of the serum level of amitriptyline. Duration of inhibition corresponds to the half-lives of the respective drugs (Deitch, 2003; Hospitalist.Net, 2005; Peterson, 2001; Sadock & Sadock, 2003).
Four concepts are important to understand regarding the metabolism and excretion of psychotropic drugs: peak plasma concentration, drug half-life, first pass effects, and clearance (Sadock & Sadock, 2003). The peak plasma concentration is defined as the greatest accumulation of the drug in the plasma. It varies depending upon the route of administration and rate of absorption of the drug. Parenteral administration of a drug generally achieves peak plasma concentration more rapidly than oral administration. Drug half-life refers to the amount of time it takes for metabolism and excretion to reduce the plasma concentration of a specific drug by half. The liver is the principal site of metabolism, and bile, feces, and urine are the primary routes of excretion. First pass effects describes the initial metabolism of an orally administered drug within the hepatic circulation and the fraction of the absorbed drug that reaches systemic circulation unmetabolized. For example, drugs administered sublingually enter the bloodstream directly and avoid first pass effects. Clearance refers to the amount of a drug excreted from the body in a specific period of time. Figure 16-2 depicts the pharmacokinetics and pharmacodynamics of psychotropic drug therapy.
Factors Affecting Pharmacodynamics and Pharmacokinetics
As the U.S. population becomes more ethnically diverse, the nurse must be knowledgeable about the client’s cultural beliefs and values regarding health and illness, as well as the client’s response to treatment, including drug therapies. The term ethnopharmacology describes the study of the effect of ethnicity on responses to prescribed medication. An awareness of characteristic distributions of genotypes (ie, genetic constitution of the client) and phenotypes (ie, observable attributes or physical manifestations of the client) that affect pharmacokinetics can determine decisions about drug choice and dosage (Deitch, 2003; Sherman, 2005.)
The goal of psychopharmacology is to achieve a desired beneficial effect with minimal, if any, adverse effects. The psychiatric–mental health nurse needs to have a basic understanding of factors affecting pharmacodynamics and pharmacokinetics. For example, most drug references list considerations such as contraindications to the use of a specific drug (eg, the presence of renal or liver impairment), precautions (eg, directions regarding the use and administration of a specific drug), and adverse effects (eg, headache, blurred vision, increased blood pressure). The potential for drug–drug and drug–food interactions and indications for use of a specific drug also are listed.
Drug Polymorphism
Drug polymorphism refers to pharmacodynamic and pharmacokinetic variations that occur on the basis of several factors such as a client’s age, gender, size, and body composition or genetic endowment (Kudzma, 1999; DeMott, 1999). For example, drug polymorphism may occur in the older adult as a result of age-related changes such as decreased absorption, hepatic function, protein binding, and renal excretion affecting the pharmacokinetics of a drug. The dosage of certain drugs may require calculation according to a client’s age and/or weight. The presence of pathophysiology (structural or functional damage to an organ or tissue), such as thyroid, cardiac, hepatic, or renal impairment, can interfere with drug metabolism or excretion and promote drug polymorphism (Sadock & Sadock, 2003; Sherman, 2005).
Environmental, cultural, and genetic factors contribute to drug polymorphism among various ethnic groups. Environmental factors such as diet influence the half-life of a drug and its metabolism. For example, among African American and Hispanic clients, treatment for hypertension (such as low-salt diets and the use of diuretics) can significantly affect the retention of lithium (Eskalith), increase lithium plasma levels, and place the client at risk for lithium toxicity. Cornmeal, a dietary staple for Hispanic clients, inhibits CYP3A4 pathway metabolism of substrates such as amitriptyline (Elavil) or haloperidol (Haldol), resulting in the need to frequently monitor and adjust drug dosages (Sherman, 2005). Smoking acts as an inducer of CYP microsomal enzyme substrates and speeds up the metabolism of antipsychotic agents. Smoking is also thought to increase the metabolism of benzodiazepines. Therefore, clients who smoke may require higher doses of these agents because of reduced plasma concentration of the drugs. The dosages of such drugs should be monitored and adjusted accordingly if the client stops smoking while under treatment (Sadock & Sadock, 2003).
Cultural factors that affect drug response include a client’s values and beliefs. For example, the frequent use of herbal and homeopathic remedies in some cultures can alter the body’s response to various drugs, placing clients at risk for drug–drug interactions. Many herbal formulations used in Asia contain a black pepper derivative that inhibits metabolism of clozapine (Clozaril) or olanzapine (Zyprexa) by CYP3A4 and CYP1A2 isoenzymes. Drug adverse effects may appear at a lower dosage. Also, clients may not inform clinicians that they are experiencing adverse effects caused by a certain drug because it is not acceptable to report symptoms. Instead, the clients may discontinue taking the prescribed medication (Sadock & Sadock, 2003; Sherman, 2005).
Finally, genetic or biologic factors in certain ethnic groups have an impact on drug efficacy. More than one third of African Americans and Asians produce reduced amounts of CYP2D6 isoenzyme and are, therefore, slow metabolizers of some atypical antipsychotics and antidepressants that are substrates for that enzyme. Because the metabolism of benzodiazepines by the CYP3A4 isoenzyme pathway is slower in Asians, they require lower initial doses of these drugs. African American clients tend to respond better to tricyclic antidepressants, phenothiazines, and anxiolytics than do European American clients. European Americans have higher rates of receptor binding for antidepressants, but respond more slowly to these drugs and
require higher doses. Asian clients respond to lower antidepressant levels (Sherman, 2005).
require higher doses. Asian clients respond to lower antidepressant levels (Sherman, 2005).
The U.S. Food and Drug Administration (FDA) recently approved the use of a pharmacogenomic diagnostic chip (AmpliChip by Roche) for genotyping. Genotyping for CYP gene variations can identify clients who will not benefit from, or may react badly to, certain psychotropic drugs. The chip provides an accurate genotype for two drug-metabolizing enzymes, 2D6 and 2C19. Examples of psychotropic drugs metabolized by 2D6 enzyme include fluoxetine (Prozac), paroxetine (Paxil), and venlafaxine (Effexor). Examples of drugs metabolized by 2C19 enzyme include amitriptyline (Elavil), citalopram (Celexa), and sertraline (Zoloft). This information can help clinicians improve the response for ultrarapid metabolizers and minimize adverse effects experienced by poor metabolizers of these substrates (Mrazek, 2005; Sherman, 2005).
Discontinuation (Withdrawal) Syndrome
Abrupt discontinuation or reduction in the dosage of a number of psychotropic drugs can precipitate a transient emergence of clinical symptoms, referred to as discontinuation (withdrawal) syndrome with rebound or relapse of original symptoms; uncomfortable new physical and psychological symptoms; or physiologic withdrawal at times (Ramaswamy, Malik, & Dewan, 2005). They can occur with tricyclic antidepressants (TCAs) and tetracyclic antidepressants, monoamine oxidase inhibitors (MAOIs), SSRIs, and other newer antidepressants. For example, discontinuation symptoms associated with TCAs are classified as physical (eg, lethargy, headache, tremor, sweating, insomnia, nausea, vomiting) or psychological (eg, irritability, anxiety/agitation, dysphoria, nightmares, paradoxical agitation). Discontinuation symptoms rarely occur with the use of MAOIs because serious side effects and dietary restrictions discourage clinicians from prescribing them. Serotonin discontinuation syndrome may occur when dosage of SSRIs such as paroxetine (Paxil), sertraline (Zoloft), or fluoxetine (Prozac) is reduced or stopped abruptly. Clinical symptoms may include affective symptoms such as agitation or dysphoria; GI symptoms such as nausea, vomiting, or diarrhea; disequilibrium symptoms such as dizziness, vertigo, or ataxia; sensory symptoms such as paresthesia or numbness; sleep disturbances; or general somatic symptoms such as lethargy, headache, or tremor. Discontinuation symptoms generally emerge within 1 to 3 days, whereas the recurrence of depressive symptoms usually occurs 2 to 3 weeks after an antidepressant is stopped. Discontinuation symptoms generally remit within a few days if the antidepressant is re-started (Ramaswamy, Malik, & Dewan, 2005).
More severe discontinuation syndromes are associated with atypical antipsychotics such as clozapine (Clozaril), mood stabilizers such as lithium (Eskalith), dopamine receptor antagonists such as haloperidol (Haldol), and benzodiazepines such as alprazolam (Xanax). Generally, these clinical symptoms are time limited. In some situations, clinical symptoms may be minimized by restarting the drug if it was discontinued abruptly and then gradually reducing the dosage. Gradual dosage reduction is recommended especially if the client received a high dosage of the drug for a period of at least 2 months (Sadock & Sadock, 2003).
Medicating the Psychiatric–Mental Health Client
According to a 1997 study of 1,228 psychiatric clients, close to 90% were treated with medication. Approximately 75% had psychiatric management (ie, the monitoring of clinical status; client and family education; the prescription, monitoring, and adjustment of medication or other treatments; the establishment of a therapeutic relationship; and the development or modification of a treatment plan). A significantly lower percentage—approximately 45%—underwent psychotherapy. The findings clearly show the psychiatric community’s current reliance on medication in treating clients (Pincus, Zarin, Tanielian, & Johnson et al., 1999).
The decision to medicate a client exhibiting clinical symptoms of a psychiatric disorder is often based on consideration of several guidelines (Bernstein, 1998). These guidelines are highlighted in Box 16-1. Some clinicians practice off-label prescribing. For example, the anticonvulsant gabapentin (Neurontin) has been prescribed for the treatment of phantom stump pain after a below-the-knee amputation, and the tetracyclic antidepressant mirtazapine (Remeron) has been administered in small doses to induce sleep. Various anticonvulsant drugs are also used to stabilize behavior in clients with the diagnosis of bipolar disorder or personality disorder. According to a nationwide survey conducted in 2004, 48% of psychiatric clients opposed off-label prescribing compared with 31% who favored
it; 22% were uncertain (Foley & Ries, 2004). Off-label prescribing is legal, common, necessary, and recognized in some states by statute and by U.S. Supreme Court review (Kramer & McCall, 2006.) Special treatment considerations are given to children, older adult clients, pregnant and nursing women, persons with hepatic or renal insufficiency, and persons with comorbid medical illnesses. However, even in the most ideal situations, psychopharmacology can result in adverse effects affecting the client’s treatment. Table 16-1 summarizes common adverse effects of psychotropic medications and appropriate nursing interventions.
it; 22% were uncertain (Foley & Ries, 2004). Off-label prescribing is legal, common, necessary, and recognized in some states by statute and by U.S. Supreme Court review (Kramer & McCall, 2006.) Special treatment considerations are given to children, older adult clients, pregnant and nursing women, persons with hepatic or renal insufficiency, and persons with comorbid medical illnesses. However, even in the most ideal situations, psychopharmacology can result in adverse effects affecting the client’s treatment. Table 16-1 summarizes common adverse effects of psychotropic medications and appropriate nursing interventions.
Box 16.1: Psychopharmacologic Guidelines: Medicating the Client
Rational pharmacotherapy requires decisions regarding when and when not to medicate.
Emotional responses to ordinary life situations should generally not be medicated.
Psychiatric illnesses such as depression and psychosis generally require pharmacotherapy.
Failure to medicate properly may prolong the client’s illness and suffering.
Irrational use of medications may lead to simultaneous adverse reactions to multiple drugs.
Detailed medical and psychiatric history, as well as assessment of client attitudes toward medication, are needed.
Appropriate medication must be carefully chosen.
The dose should be titrated according to response and adverse effects; the “standard dose” is seldom optimal.
All medications should be temporarily withheld if unexplained adverse response occurs.
It is generally advisable to start only one medication at a time, and to observe the client before others are added.
Lack of desired response may indicate that the client is not taking medication as directed.
The physician must be aware of the possible lack of bioequivalence among generic drug preparations.
The addicting potential of sedatives such as benzodiazepines should be kept in mind.
Interactions between various psychotropic drugs and between psychotropic and nonpsychotropic drugs, as well as potential interactions between alcoholic beverages and various medications, must be considered in prescribing medication.
Physicians must be aware of increased sensitivity and persistence of various drugs in elderly clients, and the interactions between underlying medical conditions and psychotropic drugs.
Before medication is prescribed, the potential risks and benefits of treatment must be discussed with the client.
Antipsychotic Agents
Antipsychotic agents are used primarily to treat most forms of psychosis, such as schizophrenia, schizoaffective disorder, delusional disorder, mood disorder with psychosis, and psychoses associated with delirium and dementia. Symptoms may include impaired communication or the inability to relate to others, delusions, hallucinations, lack of responsiveness to external stimuli, and the inability to identify reality. These agents are also used to manage confusion, behavior problems, and personality disorders. Small doses may be used to treat anxiety, tension, agitation, dizziness, intractable hiccups, nausea and vomiting, and to control pain when combined with other drugs.
Antipsychotic agents that produce significant neurologic adverse effects have been referred to as neuroleptics; the terms conventional antipsychotics and typical antipsychotics are also used to describe these agents. Antipsychotic agents provide symptom control by blocking the dopamine receptors in the brain. Dopamine is a chemical messenger that regulates thinking, emotion, behavior, and perception. Excess amounts of dopamine cause nerve impulses in the brain stem to be transmitted faster than normal, resulting in strange thoughts, hallucinations, and bizarre behavior. Blocking dopamine activity lessens or prohibits the development of such thoughts and behaviors. Examples of typical antipsychotic agents include phenothiazines (eg, thioridazine) and nonphenothiazines (eg, haloperidol).
New-generation antipsychotics, referred to as atypical antipsychotics, that block the activity of both serotonin and dopamine have been developed. Thus, they are used to treat both the positive and negative symptoms of disorders such as schizophrenia (see Chapter 22 for discussion of symptoms of schizophrenia). These agents produce fewer motor adverse effects than the typical neuroleptic agents do. Examples of
atypical antipsychotics include clozapine (Clozaril), risperidone (Risperdal), olanzapine (Zyprexa), quetiapine (Seroquel), aripiprazole (Abilify), and amisulpride (Solian).
atypical antipsychotics include clozapine (Clozaril), risperidone (Risperdal), olanzapine (Zyprexa), quetiapine (Seroquel), aripiprazole (Abilify), and amisulpride (Solian).
|
Traditionally, typical antipsychotics such as haloperidol (Haldol) are used to treat psychiatric emergency situations involving acute psychosis. However, a study published by Sherman in the October 1999 issue of the Journal of Clinical Psychiatry discusses the intramuscular use of droperidol (Inapsine), a dopamine receptor antagonist, to treat psychiatric emergencies such as acute psychosis and delirium. Researchers found that droperidol was more rapidly absorbed with intramuscular injection, was eliminated more quickly from the body, and had adverse effects comparable to, and possibly somewhat less than, haloperidol. Clients who received droperidol showed a more rapid response to the medication and were less likely to need a second injection.
Contraindications and Precautions
Contraindications to the use of antipsychotic agents include a history of drug hypersensitivity, severe depression, bone marrow depression, blood dyscrasias, and brain damage. Clients with a history of impaired liver function, cardiovascular disease, hypertension, glaucoma, diabetes, Parkinson’s disease, peptic ulcer disease, seizure disorder, or pregnancy require close observation when taking antipsychotic medication. Although most atypical antipsychotics have not been associated with prolongation of the Q–T interval as evidenced on electrocardiogram (ECG), caution is needed if these agents are prescribed with other drugs known to increase the Q–T interval. Antipsychotic agents are used cautiously in older adult clients.
General Adverse Effects
Adverse effects may occur due to the use of antipsychotic medication or due to a drug–drug interaction if the client is taking other medication to treat a medical problem. For example, delirium or neurotoxicity may occur if a client is taking medications such as haloperidol (Haldol) and diphenhydramine (Benadryl) concurrently. Clozapine (Clozaril) may cause a serious hematologic side effect called agranulocytosis. This side effect can be potentially life threatening and thus the client needs to be closely monitored with weekly blood work initially and at regular intervals thereafter. The newer-generation antipsychotics have been associated with new-onset type 2 diabetes, changes in lipid metabolism and blood concentrations, elevation of prolactin causing galactorrhea or hyperprolactinemia, and arrhythmias. Combining anxiolytics, anticonvulsants, alcohol, or antidepressants with an antipsychotic agent can induce sedation. Major adverse effects of antipsychotic drugs are listed in Box 16-2. Although the list is lengthy, most of the effects usually are mild. They can be annoying, however, and should be treated as soon as they are recognized.
Box 16.2: Major Adverse Effects of Antipsychotic Drugs
Anticholinergic effects such as drowsiness, dry mouth, nasal congestion, blurred vision, constipation, and urinary retention
Skin reactions such as urticaria, dermatitis, and pigmentation of the skin
Photosensitivity or phototoxicity
Gastrointestinal distress such as nausea, heartburn, and cholestatic jaundice
Orthostatic hypotension during the first 2 weeks of treatment
Weight gain and possible edema
Seizures due to a lowering of the seizure threshold
Alteration in sexual functioning due to diminished sex drive
Alteration in laboratory values: agranulocytosis, neutropenia, hyperglycemia, elevated prolactin, and increased cholesterol
Mild ECG changes; sinus tachycardia
Neuromuscular or Neurologic Adverse Effects
Antipsychotic drugs are associated with neuromuscular and neurologic adverse effects. The major adverse effects include acute dystonia (abnormal positioning or spasm of muscles of the head, neck, trunk, or limbs), acute dyskinesia (any disturbance of movement), parkinsonism, and akathisia (motor restlessness), which are collectively called extrapyramidal adverse effects [EPS]; tardive dyskinesia (TD); and neuroleptic malignant syndrome (NMS) (Shahrokh & Hales, 2003). A summary of these neuromuscular or neurologic adverse effects and their treatment is presented in Table 16-2. Some of the newer,
atypical antipsychotics demonstrate improved adverse effect profiles.
atypical antipsychotics demonstrate improved adverse effect profiles.
|
Extrapyramidal adverse effects may occur during the early phase of drug therapy. Acute dystonia (also referred to as tardive dystonia) is diagnosed on the basis of sustained muscle contractions that develop after at least 1 month of antipsychotic treatment, although tardive phenomena usually develop months to years after exposure to antipsychotic drugs (Koller & Cho, 2004). Tardive dyskinesia may occur after short-term use of moderate doses, although it generally occurs after long-term use of high-dose therapy. Neuroleptic malignant syndrome can occur suddenly from 1 to 7 days after neuroleptic therapy is initiated, or can occur as late as 2 months into therapy. It has also occurred when dopamine receptor (dopaminergic) agonists, such as the combination of carbidopa and levodopa (Sinemet) used for Parkinson’s disease, are discontinued (Marder & Van Putten, 1995; Herman, 1998). Clozapine (Clozaril), an atypical antipsychotic, does not cause these neuromuscular or neurologic adverse effects; often it is administered to clients who exhibit psychotic symptoms and also have neurologic disorders such as Parkinson’s disease.
Nursing Implications
Antipsychotic treatment has changed dramatically during the last 50 years. From their start as pure dopamine D2 antagonists, to serotonin/dopamine antagonists, and now to partial dopamine agonists, these medications have changed in their mechanism of action as well as expected benefits (Littrell, 2004). In 2003, the FDA asked all manufacturers of atypical antipsychotic medications to add a black box warning statement to the prescribing information stating the potential for the development of metabolic syndrome (hyperglycemia, dyslipidemia, and abdominal obesity). The risk for these three closely interlinked conditions seems to differ according to the antipsychotic drug used: the risk is the highest with the use of clozapine (Clozaril) and olanzapine (Zyprexa) (Gerson & Margolis, 2004).
Before starting therapy and at periodic intervals as determined by the prescribing clinician, clients receiving antipsychotic drug therapy need an evaluation of blood pressure, complete blood count (CBC), serum glucose level, lipid panel, liver function tests, and vision tests. They also should undergo a thorough baseline screening for personal and family history of metabolic problems, hypertension, and cardiovascular disease; assessment of body mass index (BMI) and waist circumference; and nutritional and activity counseling (American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and North American Association for the Study of Obesity, 2004).
When administering antipsychotic drugs, keep in mind the following:
If antacids are needed, administer them either 2 hours before or 1 hour after administration of the antipsychotic medication.
If a single daily dose is ordered, administer oral form within 1 or 2 hours of bedtime, whenever possible, to aid sleep. Minor adverse effects are less bothersome at this time.
Avoid contact with concentrated solutions while preparing them, because they are irritating to the skin and may cause contact dermatitis.
When administering liquid concentrates, follow pharmacy recommendations to mask the taste of the concentrate and to avoid irritation of oral mucosa.
Do not administer antipsychotic drugs subcutaneously unless specifically ordered. Change needles after filling the syringe and before injecting the medication to avoid tissue irritation. The Z-track method is generally used. Fluphenazine (Prolixin) and haloperidol (Haldol) may be injected in the deltoid or gluteal muscle. Risperidone (Risperdal) is to be injected in the gluteal muscle only.
Be aware that both olanzapine (Zyprexa [Zyprexa Zydis]) and risperidone (Risperdal-M-TAB) come in an orally disintegrating form; therefore, monitor the client’s compliance to ensure adequate absorption.
If a client is noncompliant, expect to administer the antipsychotic drug intramuscularly. Fluphenazine decanoate (Prolixin) may be administered every 1 to 2 weeks, haloperidol decanoate (Haldol LA) is usually administered every 2 to 4 weeks, and risperidone (Risperdal) is administered every 2 weeks to clients who are noncompliant regarding use of prescribed medication.
Know that antipsychotic agents may provoke seizures in clients with seizure disorders.
Closely observe the client receiving antipsychotic drugs for the following:
Therapeutic effects of the drugs, such as decreased agitation, decreased hallucinations, and increased socialization
A decrease in nausea and vomiting if the drug is administered as an antiemetic
Drug-induced EPS, early signs of TD, and NMS
Anticholinergic effects, respiratory depression, and hypersensitivity
Signs of agranulocytosis (eg, sore throat, fever, discomfort)
Drug-induced, endocrine-related changes such as menstrual irregularities, breast enlargement, lactation, hyperglycemia, and changes in libido
Signs of weight gain, jaundice, high fever, upper abdominal pain, nausea, diarrhea, and skin rash
Box 16.3: Med Alert: Antipsychotics/Neuroleptics
Dosages of neuroleptics or antipsychotic drugs are reduced when administered to children, adolescents, or the elderly. Nevertheless, drug interactions can occur. Following is a summary of drug–drug interactions to be considered when providing care to clients receiving neuroleptic drugs:
Neuroleptics can potentiate the effects of central nervous system depressants, antidepressants, anticholinergic agents, phenytoin, beta blockers, antibiotics such as tetracycline, thiazide diuretics, antihypertensives, surgical muscle relaxants, and quinidine.
Neuroleptics can reduce the effects of lithium, anticonvulsants, antibiotics, postganglionic blocking agents, antiparkinson agents, methyldopa, and hypoglycemic agents.
The effects of neuroleptics can be increased by the concomitant use of antidepressants, beta blockers, barbiturates, and methyldopa.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Get Clinical Tree app for offline access