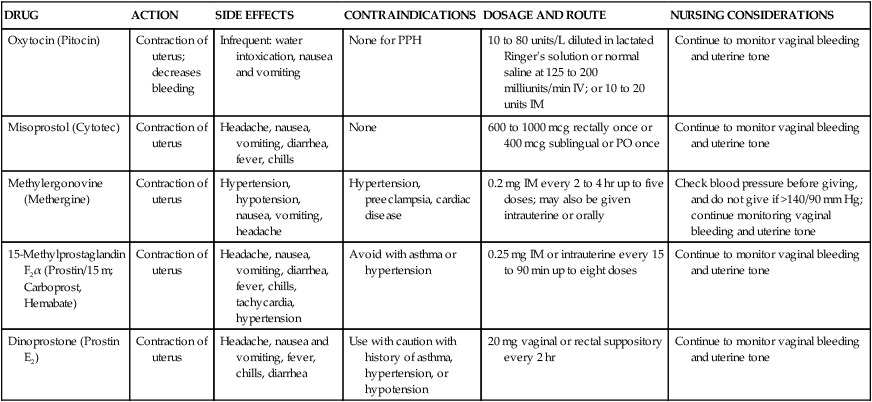
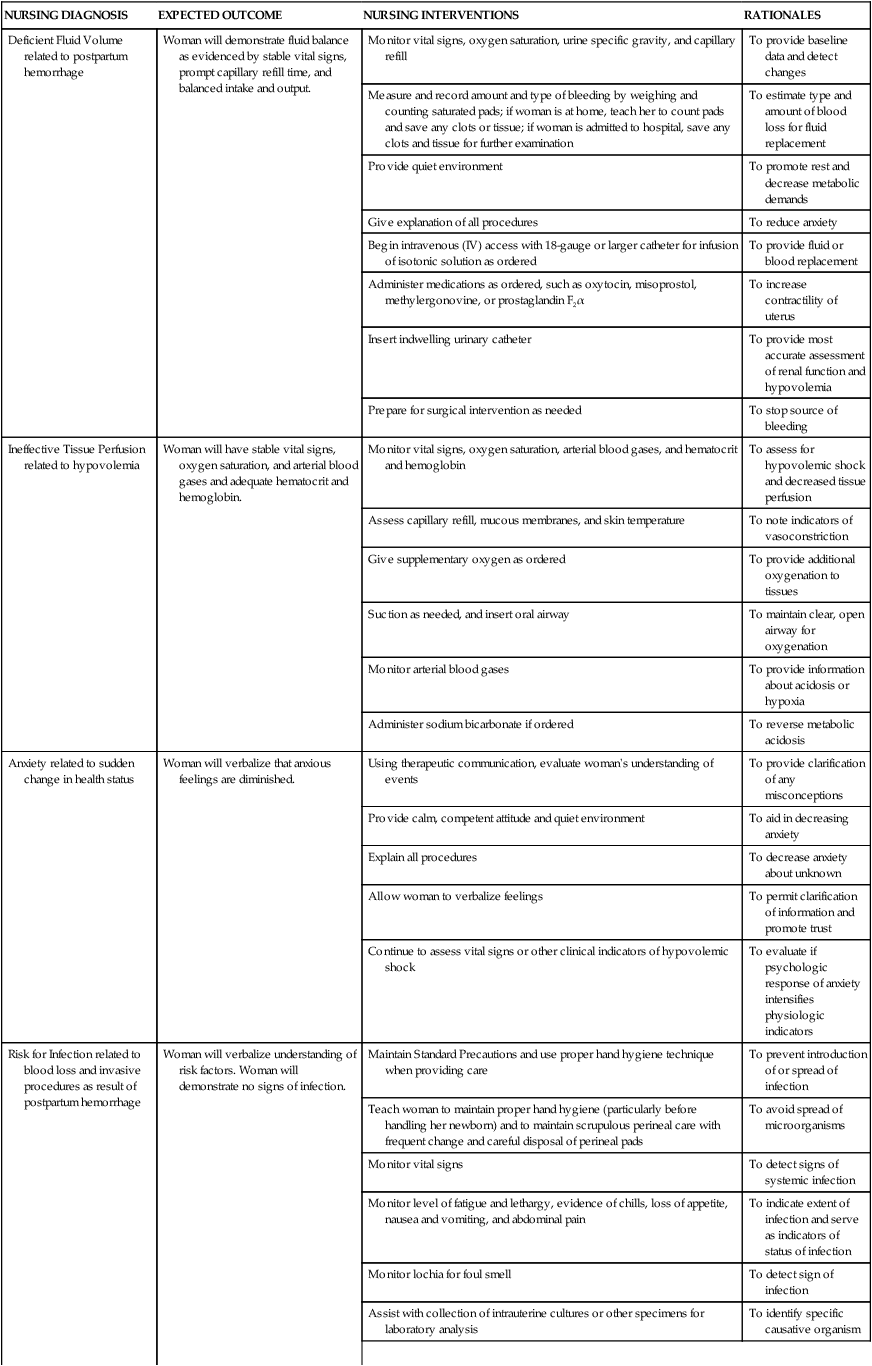
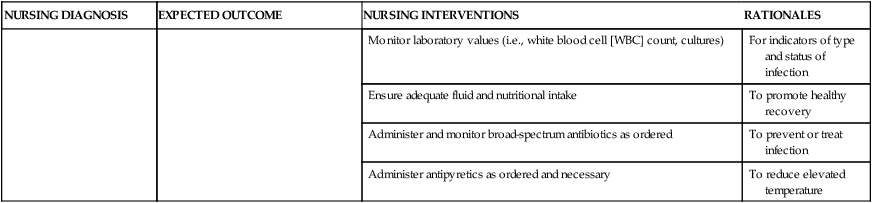
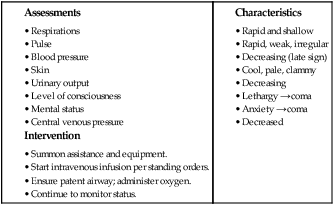
On completion of this chapter, the reader will be able to: • Identify causes, signs and symptoms, possible complications, and medical and nursing management of postpartum hemorrhage. • Describe hemorrhagic shock as a complication of postpartum hemorrhage, including medical management and nursing interventions. • Identify causes, signs and symptoms, possible complications, and medical and nursing management of postpartum infection. • Describe thromboembolic disorders, including incidence, etiology, signs and symptoms, and management. • Summarize the role of the nurse in the home setting in assessing potential problems and managing care of women with postpartum complications. • Describe structural disorders of the uterus and vagina that can result from childbearing. • Differentiate among postpartum psychologic complications, including incidence, risk factors, signs and symptoms, severity, and management. • Describe the nurse’s role in assisting families who are grieving after maternal death. Postpartum hemorrhage (PPH) is among the leading causes of maternal death in the United States and worldwide. It is a life-threatening event that can occur with little warning and is often unrecognized until the mother has profound symptoms. Postpartum hemorrhage occurs in approximately 3% of births (Callaghan, Kuklina, and Berg, 2010). It is preventable in more than half of the cases (Della Torre, Kilpatrick, Hibbard, et al., 2011). PPH is defined as the loss of 500 mL or more of blood after vaginal birth and 1000 mL or more after cesarean birth. Either a 10% change in hematocrit between admission for labor and postpartum or the need for erythrocyte transfusion is used to define PPH (Francois and Foley, 2012). However, defining PPH clinically is not a clear-cut undertaking. Diagnosis is often based on subjective observations, with blood loss often being underestimated by as much as 50% (Cunningham, Leveno, Bloom, et al., 2010). Postpartum hemorrhage is classified as early or late with respect to the birth. Early, acute, or primary PPH occurs within 24 hours of the birth. Late or secondary PPH occurs more than 24 hours but less than 6 weeks after the birth (Francois and Foley, 2012). Today’s health care environment encourages shortened hospital stays after birth, which increases the potential for acute episodes of PPH to occur outside the traditional hospital or birth center setting. Excessive bleeding can occur during the period from the separation of the placenta to its expulsion or removal. Commonly, such bleeding is the result of incomplete placental separation, undue manipulation of the fundus, or excessive traction on the cord. After the placenta has been expelled or removed, persistent or excessive blood loss usually is a result of uterine atony (i.e., failure to contract well or maintain contraction) or prolapse of the uterus into the vagina. Late PPH usually is the result of infection, subinvolution of the placental site, retained placental tissue, or coagulopathy (Francois and Foley, 2012). Risk factors for and causes of PPH are listed in Box 21-1. Uterine atony is marked hypotonia (relaxation) of the uterus. Normally, placental separation and expulsion are facilitated by contraction of the uterus, which also prevents hemorrhage from the placental site. The uterine corpus is in essence a basket weave of strong, interlacing smooth-muscle bundles through which many large maternal blood vessels pass (see Fig. 3-3). If the uterus is flaccid after detachment of all or part of the placenta, brisk venous bleeding occurs and normal coagulation of the open vasculature is impaired and continues until the uterine muscle is contracted. Uterine atony is the leading cause of early PPH. It is associated with high parity, polyhydramnios, fetal macrosomia, and multifetal gestation. In such conditions, the uterus is “overstretched” and contracts poorly after birth. Other causes of atony include traumatic birth, use of halogenated anesthetic (e.g., halothane), magnesium sulfate, rapid or prolonged labor, chorioamnionitis, use of oxytocin for labor induction or augmentation, and uterine atony in a previous pregnancy (Francois and Foley, 2012). When the placenta has not been delivered within 30 minutes after birth despite gentle traction on the umbilical cord and uterine massage, it is described as “retained.” Initial management of a retained placenta consists of manual separation and removal by the physician or nurse midwife. Supplementary anesthesia is usually not needed for women who have had regional anesthesia for birth. For other women, administration of light nitrous oxide and oxygen inhalation anesthesia or intravenous (IV) thiopental facilitates intrauterine exploration and placental separation. A tocolytic medication such as nitroglycerin IV may be given to promote uterine relaxation (Francois and Foley, 2012). After removal of a retained placenta, the woman is at continued risk for PPH and infection. In rare instances there is abnormal adherence of the placenta to the myometrium. It is unknown why this occurs, but it is thought to result from zygote implantation in an area of defective endometrium so that no zone of separation exists between the placenta and the decidua. Attempts to remove the placenta in the usual manner are unsuccessful, and laceration or perforation of the uterine wall can result, putting the woman at great risk for severe PPH and infection (Francois and Foley, 2012). • Placenta accreta—Slight penetration of myometrium • Placenta increta—Deep penetration of myometrium Placenta accreta is most common, with its incidence increasing in association with the rise in cesarean birth rates (Cunningham, Leveno, Bloom, et al., 2010). Other risk factors include placenta previa, prior uterine surgery, endometrial defects, submucosal fibroids, parity, and maternal age (Francois and Foley, 2012). Placenta accreta can be diagnosed before birth using ultrasound and MRI, but often it is not recognized until there is excessive bleeding after birth. Bleeding with complete or total placenta accreta may not occur unless separation of the placenta is attempted. With more extensive involvement, bleeding becomes profuse when delivery of the placenta is attempted. Less blood is lost if the diagnosis is made antenatally and no attempt is made to manually remove the placenta. Treatment includes blood component replacement therapy. Hysterectomy can be indicated if bleeding is uncontrolled (Cunningham, Leveno, Bloom, et al., 2010). Lacerations of the perineum are the most common of all injuries in the lower portion of the genital tract. These are classified as first, second, third, and fourth degree (see Chapter 16). An episiotomy can extend to become either a third- or fourth-degree laceration. Pelvic hematomas (i.e., a collection of blood in the connective tissue) can be vulvar, vaginal, or retroperitoneal in origin. Vulvar hematomas are the most common. Pain is the most common symptom, and most vulvar hematomas are visible. Vaginal hematomas occur more commonly in association with a forceps-assisted birth, an episiotomy, or primigravidity (Francois and Foley, 2012). Retroperitoneal hematomas are the least common but are life threatening. They are caused by laceration of one of the vessels attached to the hypogastric artery, usually associated with rupture of a cesarean scar during labor. During the postpartum period, if the woman reports persistent perineal or rectal pain or a feeling of pressure in the vagina, a careful examination is made. However, a retroperitoneal hematoma can cause minimal pain and the initial symptoms can be signs of shock (Francois and Foley, 2012). Inversion (turning inside out) of the uterus after birth is a potentially life-threatening complication. The incidence of uterine inversion is approximately 1 in 3000 births (Cunningham, Leveno, Bloom, et al., 2010) and can recur with a subsequent birth. Uterine inversion can be incomplete, complete, or prolapsed. Incomplete inversion cannot be seen; a smooth mass can be palpated through the dilated cervix. In complete inversion, the lining of the fundus crosses through the cervical os and forms a mass in the vagina. Prolapsed inversion of the uterus is obvious—a large, red, rounded mass (perhaps with the placenta attached) protrudes 20 to 30 cm outside the introitus. Contributing factors to uterine inversion include fundal implantation of the placenta, vigorous fundal pressure, excessive traction applied to the cord, fetal macrosomia, short umbilical cord, tocolysis, prolonged labor, uterine atony, nulliparity, and abnormally adherent placental tissue (Francois and Foley, 2012). The primary presenting signs of uterine inversion are sudden and include hemorrhage, shock, and pain. The uterus is not palpable abdominally. The uterus must be replaced into its proper position by the physician or nurse midwife. Uterine inversion is an emergency situation requiring immediate interventions that include maternal fluid resuscitation, replacement of the uterus within the pelvic cavity, and correction of associated clinical conditions. Tocolytics or halogenated anesthetics may be given to relax the uterus before attempting replacement (Francois and Foley, 2012). Oxytocic agents are administered after the uterus is repositioned; broad-spectrum antibiotics are initiated. The woman’s response to treatment is observed closely to prevent shock or fluid overload. If the uterus has been repositioned manually, care must be taken to avoid aggressive fundal massage. Treatment of subinvolution depends on the cause. Ergonovine (Ergotrate) or methylergonovine (Methergine), 0.2 mg every 3 to 4 hours for 24 to 48 hours, is often used. Dilation and curettage (D&C) may be performed to remove retained placental fragments or to debride the placental site. If the cause of subinvolution is infection, antibiotic therapy is needed (Cunningham, Leveno, Bloom, et al., 2010). The initial management of excessive postpartum bleeding due to uterine atony is firm massage of the uterine fundus. Expression of any clots in the uterus, elimination of bladder distention, and continuous IV infusion of 10 to 40 units of oxytocin added to 1000 mL of lactated Ringer’s or normal saline solution also are primary interventions. If the uterus fails to respond to oxytocin, other uterotonic medications are administered. Misoprostol (Cytotec), a synthetic prostaglandin E1 analog, is often used. An advantage is that it can be given by more than one route. Common dosages of misoprostol are 600 to 1000 mcg rectally or 400 mcg sublingually. A 0.2-mg dose of ergonovine (Ergotrate) or methylergonovine (Methergine) may be given intramuscularly to produce sustained uterine contractions; this can be repeated every 2 to 4 hours. A 0.25-mg dose of a derivative of prostaglandin F2α (carboprost tromethamine [Carboprost; Hemabate]) may be given intramuscularly. It can also be given intramyometrially at cesarean birth or intraabdominally after vaginal birth. Carboprost can be repeated in recurrent doses of 0.25 mg every 15 to 90 minutes, up to 8 doses. Women with a history of asthma should not receive this medication because it can cause bronchoconstriction (Francois and Foley, 2012). Prostaglandin E2 (Dinoprostone) 20-mg vaginal or rectal suppository can be used for postpartum hemorrhage (see the Medication Guide for a comparison of uterotonic drugs used to manage PPH). In addition to the medications used to contract the uterus, rapid administration of crystalloid solutions or blood or blood products or both will be needed to restore the woman’s intravascular volume (Francois and Foley, 2012). (See Evidence-Based Practice box.) Oxygen can be given by nonrebreather facemask to enhance oxygen delivery to the cells. An indwelling urinary catheter is usually inserted to monitor urine output as a measure of intravascular volume. Laboratory studies usually include a complete blood count with platelet count, fibrinogen, fibrin split products, prothrombin time, and partial thromboplastin time. Blood type and antibody screen are done if not previously performed (Cunningham, Leveno, Bloom, et al., 2010). If bleeding persists, bimanual compression may be performed by the obstetrician or nurse-midwife. This procedure involves inserting a fist into the vagina and pressing the knuckles against the anterior side of the uterus and then placing the other hand on the abdomen and massaging the posterior uterus with it. If the uterus still does not become firm, the physician or midwife performs manual exploration of the uterine cavity for retained placental fragments. If the preceding procedures are ineffective, surgical management is needed. Surgical management options include uterine tamponade (uterine packing or an intrauterine tamponade balloon), bilateral uterine artery ligation, ligation of utero-ovarian arteries and infundibulopelvic vessels, and selective arterial embolization. Uterine compression suturing (using, for example, B-Lynch or Hayman vertical sutures) may be performed and is sometimes combined with a tamponade balloon. If other treatment measures are ineffective, hysterectomy will likely be needed (Cunningham, Leveno, Bloom, et al., 2010; Francois and Foley, 2012). The nurse must be alert to the symptoms of hemorrhage and hypovolemic shock and be prepared to act quickly to minimize blood loss (Fig. 21-1). Astute assessment of circulatory status can be done with noninvasive monitoring (Box 21-2). Interventions are based on the cause of PPH as previously discussed. Once the woman’s condition is stabilized, preparations for discharge are made. Discharge instructions for the woman who experienced PPH are similar to those for any postpartum woman. In addition, the woman should be told that she will probably feel fatigue, even exhaustion, and will need to limit her physical activities to conserve her strength. She may need instructions in increasing her dietary iron and protein intake and iron supplementation to rebuild lost red blood cell (RBC) volume. She may need assistance with infant care and household activities until she has regained strength. Some women have problems with delayed lactation or insufficient milk production and postpartum depression (PPD). Referrals for home care follow-up or to community resources may be needed, such as to Postpartum Support International at www.chss.iup.edu/postpartum (see Nursing Care Plan). Hemorrhage can result in hemorrhagic (hypovolemic) shock. Shock is an emergency situation in which the perfusion of body organs can become severely compromised and death can occur. Physiologic compensatory mechanisms are activated in response to hemorrhage. The adrenal glands release catecholamines, causing arterioles and venules in the skin, lungs, gastrointestinal tract, liver, and kidneys to constrict. The available blood flow is diverted to the brain and heart and away from other organs, including the uterus. If shock is prolonged, the continued reduction in cellular oxygenation results in an accumulation of lactic acid and acidosis (from anaerobic glucose metabolism). Acidosis (lowered serum pH) causes arteriolar vasodilation; venule vasoconstriction persists. A circular pattern is established (i.e., decreased perfusion, increased tissue anoxia and acidosis, edema formation, and pooling of blood further decrease the perfusion). Cellular death occurs. See the Emergency box for assessments and interventions for hemorrhagic shock. Vigorous treatment is necessary to prevent adverse outcomes. Management of hypovolemic shock involves restoring circulating blood volume and eliminating the cause of the hemorrhage (e.g., lacerations, uterine atony, or inversion). Critical to successful management of the woman with a hemorrhagic complication is establishment of venous access, preferably with a large-bore IV catheter. The establishment of two IV lines facilitates fluid resuscitation. Fluid resuscitation includes the administration of crystalloids (lactated Ringer’s, normal saline solution), colloids (albumin), blood, and blood components. To restore circulating blood volume, a rapid IV infusion of crystalloid solution is given at a rate of 3 mL infused for every 1 mL of estimated blood loss (e.g., 3000 mL infused for 1000 mL of blood loss). Packed red blood cells (RBCs) are usually infused if the woman is still actively bleeding and no improvement in her condition is noted after the initial crystalloid infusion. Infusion of fresh frozen plasma may be needed if clotting factors and platelet counts are below normal values (Cunningham, Leveno, Bloom, et al., 2010; Francois and Foley, 2012). The nurse continues to monitor the woman’s pulse and blood pressure. If invasive hemodynamic monitoring is ordered, the nurse may assist with placement of a central venous pressure (CVP) or pulmonary artery (Swan-Ganz) catheter. Subsequently, the nurse monitors CVP, pulmonary artery pressure, or pulmonary artery wedge pressure as ordered (Gilbert, 2011). Oxygen is administered, preferably by a nonrebreather facemask, at 10 to 12 L/min to maintain oxygen saturation. Oxygen saturation should be monitored with a pulse oximeter, although measurements are not always accurate in a patient with hypovolemia or decreased perfusion. Level of consciousness is assessed frequently and provides additional indications of blood volume and oxygen saturation (Gilbert, 2011). In early stages of decreased blood flow, the woman may report “seeing stars” or feeling dizzy or nauseated. She can become restless and orthopneic. As cerebral hypoxia increases, she can become confused and react slowly to stimuli or not at all. Some women complain of headaches. An improved sensorium is an indicator of improved perfusion. Continuous electrocardiographic monitoring may be indicated for the woman who is hypotensive or tachycardic, continues to bleed profusely, or is in shock. A Foley catheter is inserted and a urometer is attached to allow hourly assessment of urine output. The most objective and least invasive assessment of adequate organ perfusion and oxygenation is a urine output of at least 30 mL/hr (Cunningham, Leveno, Bloom, et al., 2010). Hemoglobin and hematocrit levels, platelet count, and coagulation studies are closely monitored. Idiopathic or immune thrombocytopenic purpura (ITP) is an autoimmune disorder in which antiplatelet antibodies decrease the life span of the platelets. Thrombocytopenia, capillary fragility, and increased bleeding time are diagnostic findings. ITP can cause severe hemorrhage after cesarean birth or cervical or vaginal lacerations. The incidence of postpartum uterine bleeding and vaginal hematomas is also increased. Neonatal thrombocytopenia can result, but serious bleeding is unusual (Rozance and Rosenberg, 2012). Medical management focuses on control of platelet stability. If ITP was diagnosed during pregnancy, the woman likely was treated with corticosteroids or IV immune globulin. Platelet transfusions are usually given when there is significant bleeding. A splenectomy may be needed if the ITP does not respond to medical management (Cunningham, Leveno, Bloom, et al., 2010). von Willebrand disease (vWD), a type of hemophilia, is probably the most common of all hereditary bleeding disorders. Although von Willebrand disease is rare, it is among the most common congenital clotting defects in U.S. women of childbearing age. It results from a deficiency or defect in a blood clotting protein called von Willebrand factor (vWF). There are as many as 20 variations of vWD, most of which are inherited as autosomal dominant traits—types I and II are the most common (Cunningham, Leveno, Bloom, et al., 2010). Symptoms include recurrent bleeding episodes such as nosebleeds or after tooth extraction, bruising easily, prolonged bleeding time (the most important test), factor VIII deficiency (mild to moderate), and bleeding from mucous membranes. Although factor VIII increases during pregnancy, a risk for PPH still exists as levels of vWF begin to decrease (Cunningham, Leveno, Bloom, et al., 2010). The woman can be at risk for bleeding for up to 4 weeks after birth. The treatment of choice is administration of desmopressin, which promotes the release of vWF and factor VIII. It can be given nasally, intravenously, or orally. Transfusion therapy with plasma products that have been treated for viruses and contain factor VIII and vWF also may be used. Concentrates of antihemophiliac factor (Humate-P or Alphanate) can be administered (Cunningham, Leveno, Bloom, et al., 2010). Disseminated intravascular coagulation (DIC), also known as consumptive coagulopathy, is an imbalance between the body’s clotting and fibrinolytic systems. It is a pathologic form of clotting that is diffuse and consumes large amounts of clotting factors, including platelets, fibrinogen, prothrombin, and factors V and VII. Widespread external bleeding, internal bleeding, or both can result. DIC also causes vascular occlusion of small vessels resulting from small clots forming in the microcirculation. In the obstetric population, DIC can occur as a result of acute antepartum or postpartum hemorrhage, abruptio placentae, amniotic fluid embolism, dead fetus syndrome (i.e., fetus dies but is retained in utero for at least 6 weeks), severe preeclampsia, sepsis, saline abortion, and acute fatty liver of pregnancy (Francois and Foley, 2012). Primary medical management in all cases of DIC involves correction of the underlying cause (e.g., removal of the dead fetus, treatment of existing infection or of preeclampsia or eclampsia, or removal of a placental abruption). Volume replacement, blood component therapy, optimization of oxygenation and perfusion status, and continued reassessment of laboratory parameters are the usual forms of treatment. Resolution of DIC usually begins with the birth of the neonate (Francois and Foley, 2012).
Postpartum Complications
Postpartum Hemorrhage
Definition and Incidence
Etiology and Risk Factors
Uterine Atony
Retained Placenta
Lacerations of the Genital Tract
Hematomas
Inversion of the Uterus
Subinvolution of the Uterus
Care Management
Nursing Interventions
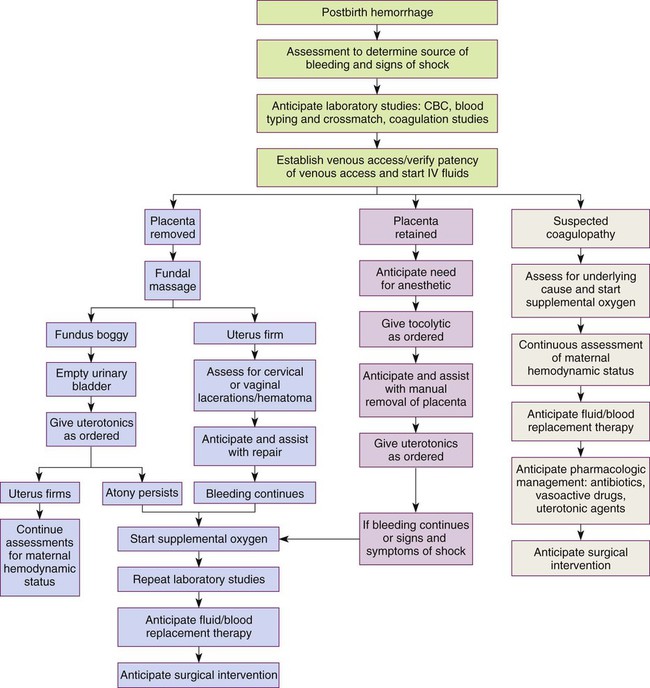
Hemorrhagic (Hypovolemic) Shock
Medical Management
Nursing Interventions
Coagulopathies
Idiopathic Thrombocytopenic Purpura
von Willebrand Disease
Disseminated Intravascular Coagulation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree