Chapter 22 On completion of this chapter, the reader will be able to: • Discuss the physiologic adaptations that the neonate must make during the period of transition from the intrauterine to the extrauterine environment. • Describe the behavioral adaptations that are characteristic of the newborn during the transition period. • Explain the mechanisms of thermoregulation in the neonate and the potential consequences of hypothermia and hyperthermia. • Recognize newborn reflexes and differentiate characteristic responses from abnormal responses. • Discuss the sensory and perceptual functioning of the neonate. • Identify signs that the neonate is at risk related to problems with each body system. At term the lungs hold approximately 20 mL of fluid per kilogram. Air must be substituted for the fluid that filled the fetal respiratory tract. Traditionally it had been thought that the thoracic squeeze occurring during normal vaginal birth resulted in significant clearance of lung fluid. However, it appears that this event plays a minor role. In the days preceding labor there is reduced production of fetal lung fluid and concomitant decreased alveolar fluid volume. Shortly before the onset of labor there is a catecholamine surge that seems to promote fluid clearance from the lungs, which continues during labor (Goldsmith, 2011). The movement of lung fluid from the air spaces occurs through active transport into the interstitium, with drainage occurring through the pulmonary circulation and lymphatic system. Retention of lung fluid can interfere with the infant’s ability to maintain adequate oxygenation, especially if other factors (e.g., meconium aspiration, congenital diaphragmatic hernia, esophageal atresia with fistula, choanal atresia, congenital cardiac defect, immature alveoli) that compromise respirations are present. Infants born by cesarean in which labor did not occur before birth can experience some lung fluid retention, although it typically clears without deleterious effects on the infant. These infants are also more likely to develop transient tachypnea of the newborn (TTNB) caused by the lower levels of catecholamines (Abu-Shaweesh, 2011). In most newborn infants auscultation of the chest reveals loud, clear breath sounds that seem very near because little chest tissue intervenes. Breath sounds should be clear and equal bilaterally. The ribs of the infant articulate with the spine at a horizontal rather than a downward slope; consequently the rib cage cannot expand with inspiration as readily as that of an adult. Because neonatal respiratory function is largely a matter of diaphragmatic contraction, abdominal breathing is characteristic of newborns. The newborn infant’s chest and abdomen rise simultaneously with inspiration. Characteristics of the respiratory system of the neonate and the effects of these characteristics on respiratory function are listed in Table 22-1. TABLE 22-1 Characteristics of the Respiratory System of the Neonate Pco2, Partial pressure of carbon dioxide. From Blackburn S: Maternal, fetal, and neonatal physiology: a clinical perspective, ed 4, St Louis, 2013, Saunders. Changes in the infant’s color can indicate respiratory distress. Acrocyanosis, the bluish discoloration of hands and feet, is a normal finding in the first 24 hours after birth. Transient periods of duskiness while crying are not uncommon immediately after birth; however, central cyanosis is abnormal and signifies hypoxemia. With central cyanosis the lips and mucous membranes are bluish. It can be the result of inadequate delivery of oxygen to the alveoli, poor perfusion of the lungs that inhibits gas exchange, or cardiac dysfunction. Because central cyanosis is a late sign of distress, newborns usually have significant hypoxemia when cyanosis appears (Askin, 2009). In neonates with more serious respiratory problems, symptoms of distress are more pronounced and tend to last beyond the first 2 hours after birth. Respiratory rates can exceed 120 breaths/min. Moderate-to-severe retractions, grunting, pallor, and central cyanosis can occur. The respiratory symptoms can be accompanied by hypotension, temperature instability, hypoglycemia, acidosis, and signs of cardiac problems. Common respiratory complications affecting neonates include RDS, meconium aspiration, pneumonia, and persistent pulmonary hypertension of the newborn (PPHN) (Askin, 2009) (see Chapter 25.) The umbilical vein and arteries constrict rapidly within the first 2 minutes after birth. It is thought that this is related to exposure of the cord to the cooler extrauterine environment and to increased oxygenation as the infant begins to breathe. With the clamping and severing of the cord, the umbilical arteries, the umbilical vein, and the ductus venosus are functionally closed; they are converted into ligaments within 2 to 3 months. The hypogastric arteries also occlude and become ligaments. Table 22-2 summarizes the cardiovascular changes at birth. TABLE 22-2 Cardiovascular Changes at Birth Data from Blackburn S: Maternal, fetal, and neonatal physiology: a clinical perspective, ed 4, St Louis, 2013, Saunders. The heart rate for a term newborn ranges from 120 to 160 beats/min, with brief fluctuations above and below these values usually noted during sleeping and waking states (Blackburn, 2013). The range of the heart rate in the term infant is about 85 to 100 beats/min during deep sleep and can increase to 180 beats/min or higher when the infant cries. A heart rate that is either high (more than 160 beats/min) or low (fewer than 100 beats/min) should be reevaluated within 30 minutes to 1 hour or when the activity of the infant changes. Immediately after birth the heart rate can be palpated by grasping the base of the umbilical cord. Apical pulse rates should be determined for all infants. Auscultation should be for a full minute, preferably when the infant is asleep. An irregular heart rate in newborns is not uncommon in the first few hours of life. After this time an irregular heart rate not attributed to changes in activity or respiratory pattern should be evaluated. Heart sounds during the neonatal period are of higher pitch, shorter duration, and greater intensity than during adult life. The first sound (S1) is typically louder and duller than the second sound (S2), which is sharp. The third and fourth heart sounds are not auscultated in newborns. Most heart murmurs heard during the neonatal period have no pathologic significance, and more than half of the murmurs disappear by 6 months. However, the presence of a murmur and accompanying signs such as poor feeding, apnea, cyanosis, or pallor are considered abnormal and should be investigated. There can be significant cardiac defects without symptoms in the early newborn period. This reinforces the importance of ongoing assessment (Sadowski, 2010). Policies on routine assessment of neonatal BP vary. In many agencies, unless a specific indication exists, BP is not measured in the newborn on a routine basis except as a baseline. In some institutions nurses obtain four extremity BPs in the presence of any cardiovascular symptoms such as tachycardia, murmur, abnormal pulses, poor perfusion, or abnormal precordial activity. If the systolic pressure is more than 10 mm Hg higher in the upper extremities than in the lower extremities, further diagnostic testing may be needed (Kenney, Hoover, Williams, et al., 2011). Early or delayed clamping of the umbilical cord changes the circulatory dynamics of the newborn. Delayed clamping expands the blood volume from the so-called placental transfusion of blood to the newborn. Delayed cord clamping (≥2 minutes after birth) has been reported to be beneficial in improving hematocrit and iron status and decreasing anemia; such benefits can last up to 6 months (Andersson, Hellström-Westas, Andersson, et al., 2011; Arca, Botet, Palacio, et al., 2010). Polycythemia that occurs with delayed clamping is usually not harmful, although there can be an increased risk of jaundice that requires phototherapy. The newborn’s skin color can reflect cardiovascular problems. Pallor in the immediate post birth period is often symptomatic of underlying problems such as anemia or marked peripheral vasoconstriction as a result of intrapartum asphyxia or sepsis. Any prolonged cyanosis other than in the hands or feet can indicate respiratory and/or cardiac problems. The presence of jaundice can indicate ABO or Rh factor incompatibility problems (see Chapter 25). Congenital heart defects are the most common type of congenital malformations (see Chapter 36). Although the more serious defects such as tetralogy of Fallot are likely to have clinical manifestations such as cyanosis, dyspnea, and hypoxia, others such as small ventricular septal defects can be asymptomatic. The prenatal history can provide information regarding risk factors for congenital heart defects so the nurse knows to be more alert for symptoms. Maternal illness such as rubella, metabolic disease such as diabetes, and drug ingestion are associated with an increased risk of cardiac defects. Because fetal circulation is less efficient at oxygen exchange than the lungs, the fetus needs additional RBCs for transport of oxygen in utero. Therefore at birth the average levels of RBCs, hemoglobin, and hematocrit are higher than those in the adult; these levels fall slowly over the first month. At birth the RBC count ranges from 4.6 to 5.2 million/mm3 (Blackburn, 2013). The term newborn can have a hemoglobin concentration of 13.7 to 20.1 g/dL at birth, decreasing gradually to 12 to 20 g/dL during the first 2 weeks (Pagana and Pagana, 2009). Hematocrit levels at birth range from 51% to 56%, increase slightly in the first few hours or days as fluid shifts from intravascular to interstitial spaces (Blackburn, 2013), and by 8 weeks are between 39% and 59% (Pagana and Pagana, 2009). Polycythemia (central venous hematocrit greater than 65%) can occur in term and preterm infants as a result of delayed cord clamping, maternal hypertension or diabetes, or intrauterine growth restriction. Leukocytosis, with a white blood cell (WBC) count of approximately 18,000/mm3 (range 9000 to 30,000/mm3), is normal at birth (Pagana and Pagana, 2009). The number of WBCs increases to 23,000 to 24,000/mm3 during the first day after birth. The initial high WBC count of the newborn decreases rapidly, and a stable level of 12,000/mm3 is normally maintained during the neonatal period. Serious infection is not tolerated well by the newborn; leukocytes are slow to recognize foreign protein and localize and fight infection early in life. Sepsis may be accompanied by a concomitant rise in neutrophils; however, some infants initially may be seen with clinical signs of sepsis without a significant elevation in WBCs. In addition, events other than infection (i.e., prolonged crying, maternal hypertension, asymptomatic hypoglycemia, hemolytic disease, meconium aspiration syndrome, labor induction with oxytocin, surgery, difficult labor, high altitude, and maternal fever) can cause neutrophilia in the newborn. Platelet count ranges between 150,000 and 300,000/mm3 and is essentially the same in newborns as in adults (Pagana and Pagana, 2009). The levels of factors II, VII, IX, and X found in the liver decrease during the first few days of life because the newborn cannot synthesize vitamin K. However, bleeding tendencies in the newborn are rare; and, unless the vitamin K deficiency is great, clotting is sufficient to prevent hemorrhage. Anatomic and physiologic characteristics of neonates place them at risk for heat loss. Newborns have a thin layer of subcutaneous fat. The blood vessels are close to the surface of the skin. Changes in environmental temperature alter the temperature of the blood, thereby influencing temperature regulation centers in the hypothalamus. Newborns have larger body surface–to–body weight (mass) ratios than do children and adults (Blackburn, 2013). 1. Convection is the flow of heat from the body surface to cooler ambient air. Because of heat loss by convection, the ambient temperature in the nursery is kept at approximately 24° C (75.2° F), and newborns in open bassinets are wrapped to protect them from the cold. A cap may be worn to decrease heat loss from the infant’s head. 2. Radiation is the loss of heat from the body surface to a cooler solid surface not in direct contact but in relative proximity. To prevent this type of loss, cribs and examining tables are placed away from outside windows, and care is taken to avoid direct air drafts. 3. Evaporation is the loss of heat that occurs when a liquid is converted to a vapor. In the newborn heat loss by evaporation occurs as a result of vaporization of moisture from the skin. This heat loss is intensified by failing to dry the newborn directly after birth or by drying the infant too slowly after a bath. The less mature the newborn, the more severe the evaporative heat loss. Evaporative heat loss, as a component of insensible water loss, is the most significant cause of heat loss in the first few days of life. 4. Conduction is the loss of heat from the body surface to cooler surfaces in direct contact. When admitted to the nursery, the newborn is placed in a warmed crib to minimize heat loss. The scales used for weighing the newborn should have a protective cover to minimize conductive heat loss. Loss of heat must be controlled to protect the infant. Control of such modes of heat loss is the basis of caregiving policies and techniques. One method for promoting thermoregulation and maternal-newborn interaction is to place the naked newborn on the mother’s bare chest and cover both with a blanket (Fig. 22-1). This skin-to-skin contact reduces conductive and radiant heat loss and enhances newborn temperature control and maternal-infant interaction (Brown and Landers, 2011). Adults are able to produce heat through shivering; however, the shivering mechanism of heat production is rarely operable in the newborn unless there is prolonged cold exposure (Blackburn, 2013). Newborns produce heat through nonshivering thermogenesis. This is accomplished primarily by metabolism of brown fat, which is unique to the newborn; and secondarily by increased metabolic activity in the brain, heart, and liver. Brown fat is located in superficial deposits in the interscapular region and axillae and in deep deposits at the thoracic inlet, along the vertebral column, and around the kidneys. Brown fat has a richer vascular and nerve supply than ordinary fat. Heat produced by intense lipid metabolic activity in brown fat can warm the newborn by increasing heat production as much as 100%. Reserves of brown fat, usually present for several weeks after birth, are rapidly depleted with cold stress. The amount of brown fat reserve increases with the weeks of gestation. A full-term newborn has greater stores than a preterm infant. The basal metabolic rate increases with cold stress. If cold stress is protracted, anaerobic glycolysis occurs, resulting in increased production of acids. Metabolic acidosis develops; and, if a defect in respiratory function is present, respiratory acidosis also develops (Fig. 22-2). Excessive fatty acids can displace the bilirubin from the albumin-binding sites and exacerbate hyperbilirubinemia. Although occurring less frequently than hypothermia, hyperthermia can occur and must be corrected. A body temperature greater than 37.5° C (99.5° F) is considered to be abnormally high and is typically caused by excess heat production related to sepsis or a decrease in heat loss. Hyperthermia can result from the inappropriate use of external heat sources such as radiant warmers, phototherapy, sunlight, increased environmental temperature, and the use of excessive clothing or blankets (Brown and Landers, 2011). The clinical appearance of the infant who is hyperthermic often indicates the causative mechanism. Infants who are overheated because of environmental factors such as being swaddled in too many blankets exhibit signs of heat-losing mechanisms: skin vessels dilate, skin appears flushed, hands and feet are warm to touch, and the infant assumes a posture of extension. The newborn who is hyperthermic because of sepsis appears stressed: vessels in the skin are constricted, color is pale, and hands and feet are cool. Hyperthermia develops more rapidly in a newborn than in an adult because of the relatively larger surface area of an infant. Sweat glands do not function well. Serious overheating of the newborn can cause cerebral damage from dehydration or even heat stroke and death (Brown and Landers, 2011). At birth a small quantity (approximately 40 mL) of urine is usually present in the bladder of a full-term infant. Many newborns void at the time of birth, although this is easily missed and may not be recorded. During the first few days term infants generally excrete 15 to 60 mL/kg; output gradually increases over the first month (Blackburn, 2013). The frequency of voiding varies from 2 to 6 times per day during the first and second days of life and from 5 to 25 times during the subsequent 24 hours. Approximately six to eight voidings per day of pale, straw-colored urine indicate adequate fluid intake. Full-term newborns have limited capacity to concentrate urine; therefore the specific gravity ranges from 1.001 to 1.020 (Pagana and Pagana, 2009). The ability to concentrate urine fully is attained by about 3 months of age. After the first voiding the infant’s urine may appear cloudy (because of mucus content) and have a much higher specific gravity. This decreases as fluid intake increases. Normal urine during early infancy is usually straw colored and almost odorless. Sometimes pink-tinged uric acid crystal stains or “brick dust” appear on the diaper; these stains are normal, although they can be misinterpreted as blood. Loss of fluid through urine, feces, lungs, increased metabolic rate, and limited fluid intake results in a 5% to 10% loss of the birth weight. This usually occurs over the first 3 to 5 days of life. If the mother is breastfeeding and her milk supply has not come in yet (which occurs by the third or fourth day after birth), the neonate is somewhat protected from dehydration by its increased extracellular fluid volume. The neonate should regain the birth weight within 10 to 14 days, depending on the feeding method (breast or bottle). In the term neonate approximately 75% of body weight consists of total body water (extracellular and intracellular). A reduction in extracellular fluid occurs with diuresis during the first few days after birth. The weight loss experienced by most newborns during the first few days after birth is caused primarily by extracellular water loss (Dell, 2011). The daily fluid requirement for neonates weighing more than 1500 g is 60 to 80 mL/kg during the first 2 days of life. From 3 to 7 days the requirement is 100 to 150 mL/kg/day; and from 8 to 30 days it is 120 to 180 mL/kg/day (Dell, 2011). The renal system has a wide range of functions. Dysfunction resulting from physiologic abnormalities can range from the lack of a steady stream of urine to gross anomalies such as hypospadias and exstrophy of the bladder, which can be identified easily at birth. Enlarged or cystic kidneys can be identified as masses during abdominal palpation. Some kidney anomalies also can be detected by ultrasound examination during pregnancy (see Chapter 25). Sucking is a reflex behavior that begins in utero as early as 15 to 16 weeks. Sucking behavior is influenced by neuromuscular maturity, maternal medications received during labor and birth, and the type of initial feeding. As early as 28 weeks some infants can coordinate sucking and swallowing while breastfeeding. Bottle-feeding infants may not coordinate sucking and swallowing until 32 to 34 weeks. A special mechanism present in healthy term newborns coordinates the breathing, sucking, and swallowing reflexes necessary for oral feeding. This is well developed in most infants by 37 weeks (Gardner and Lawrence, 2011). Sucking takes place in small bursts of 3 or 4 and up to 8 to 10 sucks at a time, with a brief pause between bursts. The infant is unable to move food from the lips to the pharynx; therefore placing the nipple (breast or bottle) well inside the baby’s mouth is necessary. Peristaltic activity in the esophagus is uncoordinated in the first few days of life. It quickly becomes a coordinated pattern in healthy full-term infants, and they swallow easily.
Physiologic and Behavioral Adaptations of the Newborn
Physiologic Adjustments
Respiratory System
Initiation of Breathing
Sensory Factors.
CHARACTERISTIC
EFFECT ON FUNCTION
Immature alveoli; decreased size and number of alveoli
Risk of respiratory insufficiency and pulmonary problems
Thicker alveolar wall; decreased alveolar surface area
Less efficient gas transport and exchange
Continued development of alveoli until childhood
Possible opportunity to reduce effects of discrete lung injury
Decreased lung elastic tissue and recoil
Decreased lung compliance requiring higher pressures and more work to expand; increased risk of atelectasis
Reduced diaphragm movement and maximal force potential
Less effective respiratory movement; difficulty generating negative intrathoracic pressures; risk of atelectasis
Tendency to nose breathe; altered position of larynx and epiglottis
Enhanced ability to synchronize swallowing and breathing; risk of airway obstruction; possibly more difficult to intubate
Small compliant airway passages with higher airway resistance; immature reflexes
Risk of airway obstruction and apnea
Increased pulmonary vascular resistance with sensitive pulmonary arterioles
Risk of ductal shunting and hypoxemia with events such as hypoxia, acidosis, hypothermia, hypoglycemia, and hypercarbia
Increased oxygen consumption
Increased respiratory rate and work of breathing; risk of hypoxia
Increased intrapulmonary right-left shunting
Increased risk of atelectasis with wasted ventilation; lower Pco2
Immaturity of pulmonary surfactant system in immature infants
Increased risk of atelectasis and respiratory distress syndrome; increased work of breathing
Immature respiratory control
Irregular respirations with periodic breathing; risk of apnea; inability to rapidly alter depth of respirations
Signs of Respiratory Distress
Cardiovascular System
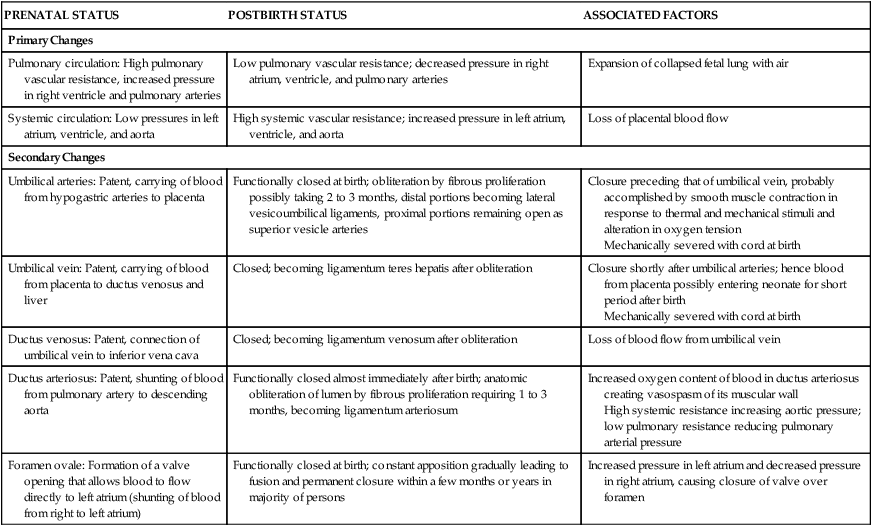
PRENATAL STATUS
POSTBIRTH STATUS
ASSOCIATED FACTORS
Primary Changes
Pulmonary circulation: High pulmonary vascular resistance, increased pressure in right ventricle and pulmonary arteries
Low pulmonary vascular resistance; decreased pressure in right atrium, ventricle, and pulmonary arteries
Expansion of collapsed fetal lung with air
Systemic circulation: Low pressures in left atrium, ventricle, and aorta
High systemic vascular resistance; increased pressure in left atrium, ventricle, and aorta
Loss of placental blood flow
Secondary Changes
Umbilical arteries: Patent, carrying of blood from hypogastric arteries to placenta
Functionally closed at birth; obliteration by fibrous proliferation possibly taking 2 to 3 months, distal portions becoming lateral vesicoumbilical ligaments, proximal portions remaining open as superior vesicle arteries
Closure preceding that of umbilical vein, probably accomplished by smooth muscle contraction in response to thermal and mechanical stimuli and alteration in oxygen tension
Mechanically severed with cord at birth
Umbilical vein: Patent, carrying of blood from placenta to ductus venosus and liver
Closed; becoming ligamentum teres hepatis after obliteration
Closure shortly after umbilical arteries; hence blood from placenta possibly entering neonate for short period after birth
Mechanically severed with cord at birth
Ductus venosus: Patent, connection of umbilical vein to inferior vena cava
Closed; becoming ligamentum venosum after obliteration
Loss of blood flow from umbilical vein
Ductus arteriosus: Patent, shunting of blood from pulmonary artery to descending aorta
Functionally closed almost immediately after birth; anatomic obliteration of lumen by fibrous proliferation requiring 1 to 3 months, becoming ligamentum arteriosum
Increased oxygen content of blood in ductus arteriosus creating vasospasm of its muscular wall
High systemic resistance increasing aortic pressure; low pulmonary resistance reducing pulmonary arterial pressure
Foramen ovale: Formation of a valve opening that allows blood to flow directly to left atrium (shunting of blood from right to left atrium)
Functionally closed at birth; constant apposition gradually leading to fusion and permanent closure within a few months or years in majority of persons
Increased pressure in left atrium and decreased pressure in right atrium, causing closure of valve over foramen
Heart Rate and Sounds
Blood Pressure
Blood Volume
Signs of Cardiovascular Problems
Hematopoietic System
Red Blood Cells and Hemoglobin
Leukocytes
Platelets
Thermogenic System
Heat Loss
Thermogenesis
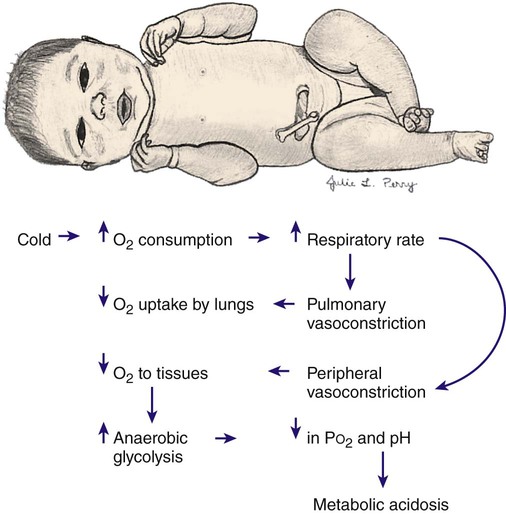
Cold Stress
Hyperthermia
Renal System
Fluid and Electrolyte Balance
Signs of Renal System Problems
Gastrointestinal System
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Physiologic and Behavioral Adaptations of the Newborn
Get Clinical Tree app for offline access

