Identify the characteristics of pediatric pharmacotherapy in children from birth to 18 years of age.
 Describe the evolution of pediatric pharmacotherapy and the purpose of federal legislation in the development of current practice standards.
Describe the evolution of pediatric pharmacotherapy and the purpose of federal legislation in the development of current practice standards.
 Describe methods for determining accurate pediatric dosing.
Describe methods for determining accurate pediatric dosing.
 Explain differences in pharmacodynamic variables between children and adults.
Explain differences in pharmacodynamic variables between children and adults.
 Explain pharmacokinetic differences between children and adults.
Explain pharmacokinetic differences between children and adults.
 Describe nursing interventions that include caregivers to help ensure safe and effective medication administration to children.
Describe nursing interventions that include caregivers to help ensure safe and effective medication administration to children.
Clinical Application Case Study
Billy Lee, a 4-year-old Native American boy, complains of shortness of breath. He is restless with expiratory wheezes. His mother reports that her son has a 2-day history of cold-like symptoms, with a productive cough and fever.
KEY TERMS
Blood–brain barrier: barrier in the central nervous system composed of capillaries with tight bonds, which acts to prevent the passage of most ions and large-molecular-weight compounds, including some drugs, from the blood to the brain
Body surface area: surface of a human body expressed in square meters
Child(ren): person(s) between birth and 18 years of age
Total body water: amount of water within the body (both intracellular and extracellular)
Introduction
Children naturally differ from adults. However, therapeutic indications and effects of drug therapy are similar in many ways. It is essential that nurses and other health care professionals understand the many ways they differ because this presents a challenge in medication dosing, administration, and management for children. For example, physiological changes throughout development influence both the pharmacodynamic and pharmacokinetic actions of medications. A child’s immature organ systems mean that molecular binding, receptor reactions, and intended actions of medications may not mimic those known for adults. Variables in absorption, distribution, metabolism, and excretion further complicate the medication process, and taken together, these differences indicate a need for vigilance in nursing management of pediatric pharmacotherapy.
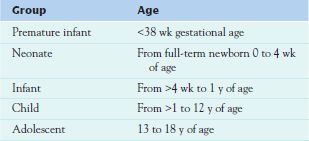
Pediatrics includes the evaluation and management of all children—patients from birth to age 18. This group is further divided into five subgroups (Table 4.1), and each developmental group is characterized by a select set of physiological changes that affect pharmacotherapy. The younger the patient, the greater the variation in medication action. Of course, many pediatric patients cannot verbalize adverse effects, and good assessment skills are crucial. It is also important to remember that as patients go through puberty, they begin to respond more like adults physiologically, but they are still immature psychologically and may lack the ability to dose, administer, or evaluate the effectiveness of medications.
Drug Safety in Pediatrics
Legislation and Drug Testing
Many challenges in pediatric medication management involve both the known physical differences in pediatric patients and the unknown action of drugs; these problems often relate to the lack of adequate information. Historically, researchers used only adults to test medications, and prescribers simply assumed that smaller doses would elicit the same results in smaller patients. However, since 1994, the process began to change. In 1994, the U.S. Food and Drug Administration (FDA) enacted the Pediatric Rule, which compelled the pharmaceutical industry to submit all known data about the pharmacokinetics, safety, and efficacy of medications used for children (Zajicek, 2009). The FDA continued the trend toward safer medication management for children when it passed the U.S. Food and Drug Administration Modernization Act of 1997 (FDAMA) followed by the 2002 Best Pharmaceuticals for Children Act (BPCA). These acts provide incentives to companies who perform research to determine the safety, efficacy, dosage, and unique risks associated with medications for children (USFDA, 2009).
The FDA continued to press for more research and better practice standards by issuing the Pediatric Research Equity Act of 2003 and renewing the BPCA in 2007. Since these measures were put in place, all the research has now uncovered other gaps in pediatric studies, including a need for pediatric formulations, preclinical studies, and pediatric outcome measures. With the current emphasis on increased patient safety and decreased medication-related adverse events, it is especially important for pediatric practitioners to be at the forefront of current research and practice standards in pediatric pharmacotherapy. Prescribers must continue to treat pediatric patients with drugs for which they lack information; therefore, they must practice good assessment, dosing, and evaluation during the administration of any medication to a pediatric patient.
Clinical Application 4-1
 Are Billy’s medications chosen based on empiric evidence obtained from solid research performed on other 4-year-old Native American boys?
Are Billy’s medications chosen based on empiric evidence obtained from solid research performed on other 4-year-old Native American boys?
Calculating Drug Dosages
The basis of pediatric drug dosing is weight, and determining drug dosages is highly dependent on the growth and development changes that occur across the lifespan. The prescriber uses weight alone to calculate pediatric dosages in an expression such as gentamicin 5 mg/kg/24 hours or determines the body surface area (BSA), the surface of a human body expressed in square meters, using the child’s weight (Mosteller, 1987) (Table 4.2). Then the prescriber calculates the dose based on a known adult dose by using the following equation: pediatric dose = BSA/1.73 × adult dose.
Pharmacodynamics in Pediatrics
Pharmacodynamics involves drug actions on target cells and the resulting alterations in cellular reactions and functions. These actions occur because of chemicals that bind with receptors at the cellular level. Most of these receptors are proteins on the surface or within cells. Therefore, pharmacodynamic variables in pediatric patients are related to differences in target cell sites and changing numbers of protein receptors. Immature organ systems and changing body compositions mean that drugs affect children differently. Causes of pharmacodynamic variability across the lifespan include differences in body composition, immature systems, and genetic makeup. Total body water, fat stores, and protein amounts change throughout childhood and greatly influence the effectiveness of drugs in the pediatric population.
One example of a drug that has different pharmacodynamic actions in adults and children is the fetal disaster caused by the drug thalidomide in the 1960s. Thalidomide worked wonders for decreasing morning sickness in pregnant women. However, it was soon apparent that it had severe teratogenic (adverse) effects on the fetus. This antiemetic drug, which was used to prevent vomiting in pregnant women, resulted in tragic limb abnormalities and often death for the fetus. Pharmacodynamic differences between drug action in adult females and their unborn fetuses illustrate one of the many differences between medication management in adults and children.
Another example of pharmacodynamic problems in younger patients involves antidepressants. Initially, prescribers assumed that these drugs, widely used successfully in treating adults, could be safely used therapeutically in adolescents and children. However, by October 2004, the FDA had found that these medications needed a BLACK BOX WARNING ♦ stating that antidepressants may play a causal role in inducing suicidality in pediatric patients (Singh et al., 2009, p. 30).
Clinical Application 4-2
 What nursing interventions would take priority in Billy’s care?
What nursing interventions would take priority in Billy’s care?
NCLEX Success
1. Billy’s medications should be individualized to ensure the best outcome. Individualizing drug therapy for a child involves which one of the following?
A. Assessing the child’s age and development level
B. Administering an adult drug selection and dosage and observing for adverse reactions
C. Deferring treatment until definitive pediatric dosing can be determined
D. Determining the child’s diet and exercise needs
2. Billy is unable to tolerate montelukast (Singulair) for his asthma, although this drug works well for his father. Billy’s physician is aware that the boy’s reaction is most likely a reflection of his
A. inability to understand the purpose of the drug
B. hope that he will not have to take the drug
C. inability to swallow pills
D. genetic variability