31 Pain management
Addiction: A chronic, neurobiologic disease characterized by impaired control over drug use, compulsive use, continued use despite harm, and craving.
Multimodal Analgesia: Combinations of drugs with different underlying mechanisms administered to allow lower doses of each of the drugs, reduce the potential for analgesic adverse effects, and provide comparable or greater pain relief than can be achieved with any single analgesic.
Neuropathic Pain: Pain that results from abnormal processing of sensory input by the nervous system because of damage to the peripheral or central nervous systems or both.
Nociceptive Pain: Pain that results from the normal functioning of physiologic systems that leads to the perception of noxious stimuli (tissue injury) as being painful.
Opioid Naive: An individual who has not recently taken enough opioid on a regular enough basis to become tolerant to the effects of an opioid.
Opioid Tolerance: An individual who has taken opioids long enough at doses high enough to develop tolerance to many of the effects of the opioid, including analgesia and sedation; a timeframe for development of tolerance has not been established.
Physical Dependence: Potential for withdrawal symptoms if the opioid is abruptly stopped or an antagonist is administered; not the same as addiction.
Pseudoaddiction: A mistaken diagnosis of addiction in which the patient exhibits behaviors often seen in addictive disease, such as asking for analgesics on time or early, but actually reflect undertreated pain.
Titration: The process of adjusting the amount of the dose of an analgesic.
Tolerance: A process characterized by decreasing effects of a drug at its previous dose, or the need for a higher dose of drug to maintain an effect; not the same as addiction.
Pain is one of the most common reasons people seek health care. Despite an abundance of research and improvements in analgesics and drug delivery technology, pain continues to be undertreated and costly for patients and the health care system in general.1,2 Nurses are experts in assessment, drug administration, and patient education and are the only members of the health care team who are at the patient’s bedside around the clock. These characteristics have led to their distinction as the patient’s primary pain manager.3 Nurses are critical to ensuring that their patients receive the best possible pain relief available.
Definition of pain
The American Pain Society defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”4 This definition describes pain as a complex, multifactoral phenomenon that affects a person’s psychosocial, emotional, and physical functioning. The definition of pain that is applied in the clinical setting reinforces that pain is a highly personal and subjective experience: “Pain is whatever the experiencing person says it is, existing whenever he says it does.”5 All accepted guidelines consider the patient’s report to be the most reliable indicator of pain and the gold standard of pain assessment.2,4
Types and categories of pain
Pain is usually described as being acute or chronic (persistent).6 Acute pain and chronic pain differ from one another primarily in their duration. For example, tissue damage as a result of surgery, trauma, or burns produces acute pain that is expected to have a relatively short duration and to resolve with normal healing. Chronic pain can occur from an underlying medical condition, such as peripheral neuropathy from diabetes, cancer pain from tumor growth, or osteoarthritis pain from joint degeneration, and it can persist throughout the course of a person’s life. Some medical conditions can produce both acute and chronic pain. For example, some patients with cancer have continuous chronic pain and also experience acute exacerbations of pain periodically (called breakthrough pain) or endure repetitive painful procedures during cancer treatment.
Pain is increasingly classified by its inferred pathology as being either nociceptive pain or neuropathic pain (Table 31-1).6 Nociceptive pain refers to the normal functioning of physiologic systems that leads to the perception of noxious stimuli (tissue injury) as being painful. This explains why nociception is described as “normal” pain transmission. Pain from surgery, trauma, burns, and tumor growth are examples of nociceptive pain. Patients often describe this type of pain as “aching,” “cramping,” or “throbbing.”
Neuropathic pain is pathologic and results from abnormal processing of sensory input by the nervous system as a result of damage to the peripheral nervous system (PNS) or central nervous system (CNS), or both.6 Examples include postherpetic neuralgia, diabetic neuropathy, phantom pain, and post-stroke pain syndrome. Patients with neuropathic pain describe their pain with distinctive words, such as “burning,” “sharp,” and “shooting.”
Nociception and analgesic action sites
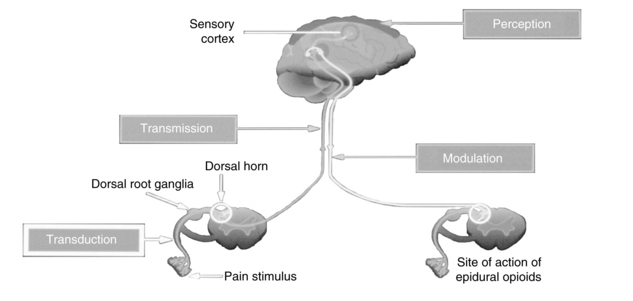
Nociception includes four specific processes: transduction, transmission, perception, and modulation. Figure 31-1 illustrates these processes, and an overview of each follows.
Transduction
Transduction refers to the processes by which noxious stimuli activate primary afferent neurons called nociceptors, which are located throughout the body in the skin, subcutaneous tissue, and visceral and somatic structures (see Fig. 31-1).6 These neurons have the ability to respond selectively to noxious stimuli generated as a result of tissue damage from mechanical (e.g., incision, tumor growth), thermal (e.g., burn, frostbite), chemical (toxins, chemotherapy), and infectious sources.7,8 The noxious stimuli cause the release of a number of excitatory compounds (e.g., serotonin, bradykinin, histamine, substance P, prostaglandins), which facilitate the movement of pain along the pain pathway.6 These substances are collectively referred to as inflammatory soup.8
Prostaglandins are a particularly important group of compounds that accompanies tissue injury and initiates inflammatory responses that increase tissue swelling and pain at the site of injury.9 They are formed when the enzyme phospholipase breaks down phospholipids into arachidonic acid, and arachidonic acid, in turn, is acted upon by the enzyme cyclooxygenase (COX) to produce prostaglandins (Fig. 31-2). The two best characterized isoenzymes of COX are COX-1 and COX-2; they have an important role in producing the effects of the nonopioid analgesics, which act peripherally and centrally to inhibit the COX isoenzymes. Nonsteroidal antiinflammatory drugs (NSAIDs) work primarily by blocking the formation of prostaglandins in the periphery. The nonselective NSAIDs, such as ibuprofen, naproxen, diclofenac, and ketorolac, inhibit both COX-1 and COX-2, and the COX-2 selective NSAIDs, such as celecoxib, inhibit just COX-2. As Fig. 31-2 illustrates, both types of NSAIDs produce antiinflammatory and pain relief through the inhibition of COX-2. Although the exact underlying mechanisms of action of acetaminophen continue to be investigated,10 acetaminophen is a known COX inhibitor that has minimal peripheral effect, is not antiinflammatory, and can both relieve pain and reduce fever by preventing the formation of prostaglandins in the CNS.6
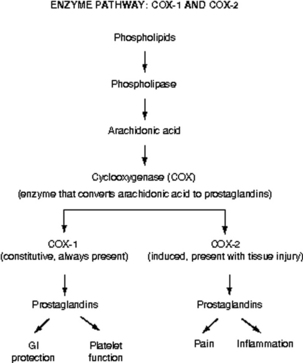
FIG. 31-2 Enzyme pathway: COX-1 and COX-2. GI, Gastrointestinal.
(From Pasero C, McCaffery M: Pain assessment and pharmacologic management, St. Louis, 2011, Mosby. Copyright Pasero C, McCaffery M. Used with permission.)
Other types of analgesics work by partially blocking transduction as well. For example, sodium channels are closed and inactive at rest, but undergo changes in response to membrane depolarization. Transient channel opening leads to an influx of sodium and subsequent nerve conduction.11 Local anesthetics are capable of blocking sodium channels and reducing the nerve’s ability to generate an action potential. Anticonvulsants also affect the flux of other ions, such as calcium and potassium, to reduce transduction and produce pain relief.
Transmission
Transmission is the second process involved in nociception. Effective transduction generates an action potential that is transmitted along the A-delta (δ) and C fibers.6 A-δ fibers are lightly myelinated and faster conducting than the unmyelinated C fibers. The endings of A-δ fibers detect thermal and mechanical injury and allow relatively quick localization of pain and a rapid reflex withdrawal from the painful stimulus. Unmyelinated C fibers are slow conductors and respond to mechanical, thermal, and chemical stimuli. They yield poorly localized, often aching or burning pain. A-beta (β) fibers are the largest of the fibers and do not normally transmit pain, but do respond to touch, movement, and vibration.6
Afferent information passes through the cell body of the dorsal root ganglia (see Fig. 31-1), which lie outside of the spinal cord, to synapse in the dorsal horn of the spinal cord. An action potential is generated and the impulse ascends up to the spinal cord and transmits information to the brain, where pain is perceived. Extensive modulation occurs in the dorsal horn via complex neurochemical mechanisms. The primary A-δ fibers and C fibers release a variety of transmitters including glutamate, neurokinin, and substance P. Glutamate binds to the N-methyl-D-aspartate (NMDA) receptor and promotes pain transmission. Ketamine is an NMDA receptor antagonist that provides pain relief by preventing glutamate from binding to the NMDA receptor sites. Endogenous and therapeutically administered opioids bind to opioid receptor sites in the dorsal horn to block substance P and thereby produce analgesia.6
Perception
The third broad process involved in nociception is perception. Perception, the result of the neural activity associated with transmission of noxious stimuli,6 involves the conscious awareness of pain and requires activation of higher brain structures for the occurrence of awareness, emotions, and drives associated with pain (see Fig. 31-1). The physiology of the perception of pain is poorly understood, but presumably can be targeted by mind-body therapies, such as distraction and imagery, which are based on the belief that brain processes can strongly influence pain perception.6
Modulation
Modulation of afferent input generated in response to noxious stimuli occurs at every level from the periphery to the cortex and involves dozens of neurochemicals.9 For example, serotonin and norepinephrine are central inhibitory neurotransmitters that are released in the spinal cord and brainstem by the descending fibers of the modulatory system (see Fig. 31-1). Some antidepressants provide pain relief by blocking the body’s reuptake of serotonin and norepinephrine, extending their availability to fight pain. Endogenous opioids are located throughout the peripheral and central nervous systems, and like therapeutically administered opioids they inhibit neuronal activity by binding to opioid receptors. As an example, Fig. 31-1 shows that the dorsal horn of the spinal cord, which is densely populated with opioid receptors, is the primary action site of epidural opioids.
Pathophysiology of neuropathic pain
Neuropathic pain is sustained by mechanisms that are driven by damage to, or dysfunction of, the PNS or CNS. In contrast to nociceptive pain, neuropathic pain is abnormal processing of stimuli.7,12 Whereas nociceptive pain involves tissue damage or inflammation, neuropathic pain can occur in the absence of either. Neuropathic pain, even when acute, reflects a pathophysiology that serves no useful purpose.6 A discussion of some of the peripheral and central mechanisms that initiate and maintain neuropathic pain follows. Extensive research is ongoing to better define these mechanisms.
Peripheral mechanisms
At any point from the periphery to the CNS, the potential exists for the development of neuropathic pain. For example, when nociceptors are injured, changes in the number and location of ion channels, particularly sodium channels, can abnormally accumulate.6 The threshold for nerve depolarization is then lowered, which leads to an increased response to stimuli and ectopic discharges. Hyperexcitable nerve endings in the periphery can become damaged, leading to abnormal reorganization of the nervous system, an underlying mechanism of some neuropathic pain states.7 Chemically mediated connections can form between nerve fibers and cause abnormal activation of neurons and ultimately pain. These processes lead to a phenomenon called peripheral sensitization, which is thought to contribute to the maintenance of neuropathic pain. Topical local anesthetics, such as lidocaine patch 5%, are an example of analgesics that produce effects in the tissues under the site of application by “dampening” neuropathic pain mechanisms in the peripheral nervous system.13
Central mechanisms
Central mechanisms also have a role in establishing neuropathic pain. Central sensitization is defined as abnormal hyperexcitability of central neurons as a result of complex changes induced by incoming barrages of nociceptors.6 Extensive release and binding of excitatory neurotransmitters, such as glutamate, activate the NMDA receptor and cause an increase in intracellular calcium levels into the neuron, resulting in pain. As noted, the NMDA antagonist ketamine directly antagonizes this activity. An increase in the influx of sodium is thought to lower the threshold for nerve activation, increase response to stimuli, and enlarge the receptive field served by the affected neuron. The accumulation of intracellular ions causes spinal neurons to become highly sensitized and fire rapidly in a process called wind-up.9 As mentioned, local anesthetics and anticonvulsants can block ion channels and inhibit abnormal pain sensation.
As with injured peripheral neurons, synaptic reorganization and anatomic changes can also occur in the CNS. These are thought to be sustained by an increased responsiveness of central neurons to relatively mild peripheral stimuli.12 For example, injury to a nerve route can lead to reorganization in the dorsal horn of the spinal cord. Nerve fibers can invade other areas and create abnormal sensations in the area of the body served by the injured nerve. Allodynia, or pain from a normally nonnoxious stimulus (e.g., touch), is one such type of abnormal sensation and a common feature of neuropathic pain. In patients with allodynia, the mere weight of clothing or bed sheets can be excruciatingly painful. The ability of the nervous system to change structure and function as a result of noxious stimuli is called neuroplasticity.6
Another underlying mechanism called central disinhibition occurs when control mechanisms along inhibitory (modulatory) pathways are lost or suppressed, leading to abnormal excitability of central neurons.6 Possible causes of disinhibition include dysfunction of the gamma-aminobutyric acid (GABA) pathways. GABA is the most abundant neurotransmitter in the CNS and composes a major inhibitory neurotransmitter system. Increased GABA function may help to relieve neuropathic pain. Benzodiazepines, such as midazolam, enhance GABA function, resulting in analgesia for pathologic conditions like muscle spasm.6,13
Harmful effects of unrelieved pain
Literally every system in the body is affected by unrelieved pain; the harmful effects are numerous (Table 31-2). Unrelieved pain triggers and prolongs the stress response causing the release of excessive amounts of hormones, such as cortisol, catecholamines, and glucagon; insulin and testosterone levels decrease.14,15 This increased endocrine activity initiates a number of metabolic processes that can result in weight loss, tachycardia, increased respiratory rate, shock, and even death.14 Persistent unrelieved pain has been linked to increased tumor growth16 and a higher incidence of health care–associated infections.17
Table 31-2 Harmful Effects of Unrelieved Pain
| DOMAINS AFFECTED | SPECIFIC RESPONSES TO PAIN |
|---|---|
| Endocrine | ↑ ACTH, ↑ cortisol, ↑ ADH, ↑ epinephrine, ↑ norepinephrine, ↑ GH, ↑ catecholamines, ↑ renin, ↑ angiotensin II, ↑ aldosterone, ↑ glucagon, ↑ interleukin-1, ↓ insulin, ↓ testosterone |
| Metabolic | Gluconeogenesis, hepatic glycogenolysis, hyperglycemia, glucose intolerance, insulin resistance, muscle protein catabolism, ↑ lipolysis |
| Cardiovascular | ↑ Heart rate, ↑ cardiac workload, ↑ peripheral vascular resistance, ↑ systemic vascular resistance, hypertension, ↑ coronary vascular resistance, ↑ myocardial oxygen consumption, hypercoagulation, deep vein thrombosis |
| Respiratory | ↓ Flows and volumes, atelectasis, shunting, hypoxemia, ↓ cough, sputum retention, infection |
| Genitourinary | ↓ Urinary output, urinary retention, fluid overload, hypokalemia |
| Gastrointestinal | ↓ Gastric and bowel motility |
| Musculoskeletal | Muscle spasm, impaired muscle function, fatigue, immobility |
| Cognitive | Reduction in cognitive function, mental confusion |
| Immune | Depression of immune response |
| Developmental | ↑ Behavioral and physiologic responses to pain, altered temperaments, higher somatization, infant distress behavior, possible altered development of the pain system, ↑ vulnerability to stress disorders, addictive behavior, and anxiety states |
| Future pain | Debilitating chronic pain syndromes: postmastectomy pain, postthoracotomy pain, phantom pain, postherpetic neuralgia |
| Quality of life | Sleeplessness, anxiety, fear, hopelessness, ↑ thoughts of suicide |
ACTH, Adrenocorticotrophic hormone; ADH, antidiuretic hormone; down arrow (↓), decreased; GH, growth hormone; up arrow (↑), increased.
From Pasero C, McCaffery M: Pain assessment and pharmacologic management, St. Louis, 2011, Mosby. Copyright Pasero C, McCaffery M. Used with permission.
Effects on the cardiovascular (CV) system include increased postoperative blood loss18 and hypercoagulation,14 which can lead to myocardial infarction and stroke. The respiratory system is affected by small tidal volumes and decreases in functional lung capacity, which can lead to pneumonia, atelectasis, and an increased need for mechanical ventilation.19,20
Every surgical procedure has the potential to produce persistent (chronic) postsurgical pain; however, inguinal hernia repair, amputation, and thoracic, cardiac, and breast surgery are among those identified as high risk for this complication.6,21,22 Multiple factors are thought to contribute to the development of persistent postsurgical pain, including nerve injury from the surgical procedure, preexisting pain, and genetic susceptibility.23 Persistent postsurgical pain may have nociceptive, inflammatory, and neuropathic components indicating a need for a multimodal treatment approach.22 Similar to other complex pain syndromes, it can be difficult to treat and last a lifetime.
Pain assessment: the gold standard
The gold standard for assessing the existence and intensity of pain is the patient’s self report.2 A comprehensive pain assessment includes obtaining the following information from the patient:
• Location of pain: Ask the patient to state or point to the areas of pain on the body.
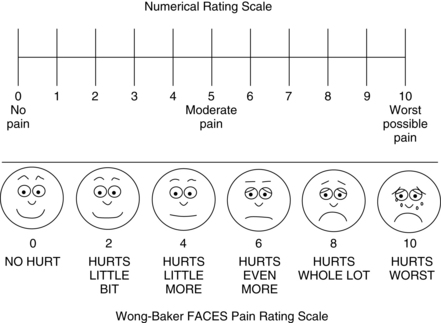
• Intensity: Ask the patient to rate the intensity of the pain using a reliable and valid pain assessment tool. A number of scales in several language translations have been evaluated and made available for use in clinical practice and for educational practice.2 See Box 31-1 for practical tips on using self-report pain rating tools. The most common are:
• Quality: Ask the patient to describe how the pain feels. Descriptors such as sharp, shooting, or burning may help to identify the presence of neuropathic pain.
• Onset and duration: Ask when the pain started and whether it is constant or intermittent.
• Aggravating and relieving factors: Ask the patient what makes the pain worse and what makes it better.
• Effect of pain on function and quality of life: It is particularly important to ask patients with persistent pain about how pain has affected their lives; what could they do before the pain began that they can no longer do, or what do they want to do but cannot do because of the pain?
• Comfort-function (pain) goal: For patients with acute pain, identify short-term functional goals and reinforce the link between good pain control and successful achievement of the goals. For example, surgical patients are told that they will be expected to cough, deep breathe, turn, and ambulate or participate in physical therapy postoperatively. Patients with chronic pain can be asked to identify their unique functional or quality-of-life goals, such as being able to work, walk the dog, or garden. Patients are then asked to identify (using a 0-to-10 scale) a level of pain that will allow accomplishment of the identified functional or quality-of-life goals with reasonable ease. A realistic goal for most patients is 2 or 3, and pain intensity ratings that are consistently above the goal warrant further evaluation and consideration of an intervention and possible adjustment of the treatment plan.2
• Other information: The patient’s culture, past pain experiences, and pertinent medical history, such as comorbidities, laboratory tests, and diagnostic studies, are considered when establishing a treatment plan.28
BOX 31-1 Practical Tips on the Use of Self-Report Pain Rating Scales
• Try using a standard pain assessment tools such as the 0-to-10 numerical rating scale; verbal descriptor scale with simple adjectives such as mild, moderate, severe, and worst possible pain; or the FACES Pain Scale-Revised.
• Increase the size of the font and other features of the scale.
• Ensure that eyeglasses and hearing aids are functioning.
• Try using alternative words, such as ache, hurt, and sore when discussing pain.
• Provide a written example of the scale and clear instructions on how to use it.
• Present the tool vertical format (rather than the frequently used horizontal).
• Ask about pain in the present.
• Repeat instructions and questions more than once.
• Allow ample time to respond.
• Ask awake and oriented ventilated patients to point to a number on the numerical scale if they are able.
• Repeat instructions and show the scale each time pain is assessed.
From Pasero C, McCaffery M: Pain assessment and pharmacologic management, St. Louis, 2011, Mosby. Copyright 2008, Pasero C, McCaffery M. Used with permission.
Challenges in assessment
Many patients are unable to provide a report of their pain using the customary self-report pain rating tools, placing them at higher risk for undertreated pain than those who can report.2 These patients are collectively called nonverbal patients29 and include infants, toddlers, and patients who are cognitively impaired, critically ill (intubated, unresponsive), comatose, or imminently dying. Patients who are receiving neuromuscular blocking agents or are sedated from anesthesia and other drugs given during surgery are also among this at-risk population.
When patients are unable to report pain using traditional methods, an alternative approach based on the Hierarchy of Pain Measures is recommended.2,29,30 The key components of the hierarchy are to: (1) attempt to obtain self-report; (2) consider underlying pathology or conditions and procedures that might be painful (e.g., surgery); (3) observe behaviors; (4) evaluate physiologic indicators; and (5) conduct an analgesic trial. See Box 31-2 for detailed information on each component of the Hierarchy of Pain Measures.
BOX 31-2 Hierarchy of Pain Measures
1. Attempt to obtain the patient’s self-report, which is the single most reliable indicator of pain. Do not assume that a patient cannot provide a report of pain; many cognitively impaired patients are able to use a self-report tool, such as the FACES Pain Scale-Revised or Verbal Descriptor Scale.
2. Consider the patient’s condition or exposure to a procedure that is thought to be painful. If appropriate, assume that pain is present and document it when approved by institution policy and procedure.
3. Observe behavioral signs such as facial expressions, crying, restlessness, and changes in activity. There are many behavioral pain assessment tools available that will yield a pain behavior score and may help to determine whether pain is present. However, it is important to remember that a behavioral score is not the same as a pain intensity score. Pain intensity is unknown if the patient is unable to provide it.
4. Evaluate physiologic indicators with the understanding that they are the least sensitive indicators of pain and may signal the existence of conditions other than pain or a lack of it (e.g., hypovolemia, blood loss). Patients may have normal or below normal vital signs in the presence of severe pain. The absence of an elevated blood pressure or heart rate does not mean the absence of pain.
5. Conduct an analgesic trial to confirm the presence of pain and to establish a basis for developing a treatment plan if pain is thought to be present. An analgesic trial involves the administration of a low dose of nonopioid or opioid and observing patient response. The initial low dose may not be enough to illicit a change in behavior and should be increased if the previous dose was tolerated, or another analgesic may be added. If behaviors continue despite optimal analgesic doses, other possible causes should be investigated. In patients who are completely unresponsive, no change in behavior will be evident and the optimized analgesic dose should be continued.
From Pasero C, McCaffery M: Pain assessment and pharmacologic management, St. Louis, 2011, Mosby. Copyright 1999, McCaffery M, Pasero C. Used with permission.
Self report is at the top of the hierarchy and should be attempted, even in patients who present challenges in assessment.2 Many patients with mild to moderate cognitive impairment can provide self-report when clinicians implement fairly simple measures (see Box 31-1).
Several behavioral pain assessment tools exist to facilitate assessment in nonverbal patients; however, patients must be carefully evaluated for their ability to respond with the requisite behaviors in the selected tool.2 For example, tools that require assessment of body movement as a pain indicator should not be used in patients who are unable to move, such as those receiving a neuromuscular blocking agent. According to the hierarchy, pain can be assumed to be present in these patients, justified by research showing that endotracheal intubation, ventilation, and suctioning—all required in patients receiving a neuromuscular blocking agent—are painful.31,32 It is equally important to understand that the score obtained from the use of a behavioral pain assessment tool helps to identify the presence of pain, but the score is a behavioral score and not a pain intensity rating. Simply put, if the patient cannot report the intensity of the pain, the intensity is not known.2,33
Although nurses who care for patients with acute pain often rely on vital signs to assess pain, these physiologic signs are considered poor indicators of pain.2,34,35 Many factors other than pain can influence changes in vital signs, and patients quickly adapt physiologically despite the presence of pain. The primary message is that the absence of an elevated blood pressure or heart rate does not mean the absence of pain.2
Reassessment of pain
Following initiation of the pain management plan, pain is reassessed and documented on a regular basis as a way to evaluate the effectiveness of treatment. At a minimum, pain should be reassessed with each new report of pain and before and after the administration of analgesics.2 The frequency of reassessment depends on the stability of the patient’s pain and is guided by institutional policy. For example, in the postanesthesia care unit (PACU) reassessment may be necessary as often as every 10 minutes when pain is unstable during opioid titration, but can be done every 4 to 8 hours in patients with stable pain 24 hours after surgery.
Pain control on A continuum
The quality of patients’ pain control should be addressed when patients are discharged from one clinical area to another. Many PACUs establish the criterion that patients must achieve a pain rating of 4 on a scale of 0 to 10 or better before discharge; however, the expectation that all patients must be discharged from a given clinical unit with pain ratings less than an arbitrary number is unrealistic and can lead to the unsafe administration of additional opioid doses to patients who are excessively sedated and is widely discouraged.28,36–38 Instead, achieving optimal pain relief is best viewed on a continuum with the primary objective being to provide both effective and safe analgesia.28 Optimal pain relief is the responsibility of every member of the health care team and begins with analgesic titration in the PACU followed by continued prompt assessment and analgesic administration after discharge from the PACU to achieve pain ratings that allow patients to meet their functional goals with relative ease.
Although it may not always be possible to achieve a patient’s pain rating goal within the short time the patient is in an area like the PACU, this goal provides direction for ongoing analgesic care. Important information to give to the nurse assuming care of the patient on the clinical unit is the patient’s pain rating goal, how close the patient is to achieving it, what has been done thus far to achieve it (analgesics and doses), and how well the patient has tolerated analgesic administration (adverse effects).28
Pharmacologic management of pain: multimodal analgesia
Pain is a complex phenomenon involving multiple underlying mechanisms as described earlier in this chapter. This characteristic underscores the importance of using more than one analgesic to manage pain.28 This approach is called multimodal analgesia and is recommended for the treatment of all types of pain.4,28,39,40 A multimodal regimen combines drugs with different underlying mechanisms; this allows lower doses of each of the drugs in the treatment plan, which reduces the potential for each to produce adverse effects.28 Furthermore, multimodal analgesia can result in comparable or greater pain relief than can be achieved with any single analgesic. The use of multimodal analgesia should be the rule, rather than the exception in pain treatment.
The most common analgesics used for postoperative pain management are nonopioid analgesics (e.g., acetaminophen, NSAIDs), opioid analgesics (e.g., morphine, hydromorphone, fentanyl, nd oxycodone), local anesthetics, and anticonvulsants. A multimodal approach in the perioperative setting may combine agents from each of these analgesic groups to provide effective pain relief and help minimize adverse effects. Unless contraindicated, all surgical patients should routinely be given acetaminophen and an NSAID in scheduled doses throughout the postoperative course (preferably initiated preoperatively). Opioid analgesics are added to manage moderate-to-severe postoperative pain in most patients. For some major surgical procedures, a local anesthetic is administered with an opioid epidurally or alone by continuous peripheral nerve block. An anticonvulsant may be added to the treatment plan as well to control severe pain or prevent a chronic postsurgical pain syndrome, such as persistent pain after thoracotomy or mastectomy.6
In a prospective, randomized-controlled study, 80 patients undergoing total knee replacement were assigned to receive morphine via intravenous (IV) patient-controlled analgesia (PCA) alone or as a single 400-mg dose of celecoxib 1 hour before surgery followed by 200 mg of celecoxib every 12 hours for 5 days postoperatively along with IV PCA morphine. Resting pain scores were better, and active range of motion increased significantly in those who received celecoxib compared with those who did not. Celecoxib demonstrated a 40% morphine dose-sparing effect, which resulted in a lower incidence of nausea and vomiting in those who received celecoxib (28%) than in those who did not (43%). Other important findings were that intraoperative and postoperative blood loss was comparable among patients in both groups and celecoxib resulted in no significant increase in the need for blood transfusions.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree