Oxygenation
Objectives
• Describe the structure and function of the cardiopulmonary system.
• Describe the physiological processes of ventilation, perfusion, and exchange of respiratory gases.
• State the process of the neural and chemical regulation of respiration.
• Discuss the effect of a patient’s level of health, age, lifestyle, and environment on oxygenation.
• Assess for the risk factors affecting a patient’s oxygenation.
• Assess for the physical manifestations that occur with alterations in oxygenation.
• Develop a plan of care for a patient with altered need for oxygenation.
Key Terms
Acute coronary syndrome (ACS), p. 828
Afterload, p. 824
Angina pectoris, p. 828
Apnea, p. 834
Atelectasis, p. 822
Bilevel positive airway pressure (BiPAP), p. 848
Bronchoscopy, p. 832
Cardiac output, p. 824
Cardiopulmonary rehabilitation, p. 854
Cardiopulmonary resuscitation (CPR), p. 853
Chest physiotherapy (CPT), p. 842
Chest tube, p. 849
Cheyne-Stokes respiration, p. 834
Continuous positive airway pressure (CPAP), p. 848
Diaphragmatic breathing, p. 854
Dyspnea, p. 831
Dysrhythmias, p. 827
Electrocardiogram (ECG), p. 825
Endotracheal (ET) tube, p. 846
Expiration, p. 822
Hematemesis, p. 832
Hemoptysis, p. 832
Hemothorax, p. 849
Humidification, p. 842
Hyperventilation, p. 827
Hypoventilation, p. 827
Hypovolemia, p. 826
Hypoxia, p. 827
Incentive spirometry, p. 848
Inspiration, p. 822
Kussmaul respiration, p. 834
Myocardial infarction (MI), p. 828
Myocardial ischemia, p. 828
Nasal cannula, p. 851
Nebulization, p. 842
Noninvasive positive-pressure ventilation (NPPV), p. 848
Normal sinus rhythm (NSR), p. 825
Orthopnea, p. 832
Perfusion, p. 822
Pneumothorax, p. 849
Postural drainage, p. 843
Preload, p. 824
Pursed-lip breathing, p. 854
Stroke volume, p. 823
Surfactant, p. 822
Tracheostomy, p. 847
Ventilation, p. 822
Ventricular tachycardia/fibrillation, p. 827
Wheezing, p. 832
![]()
Scientific Knowledge Base
Oxygen is necessary to sustain life. The cardiac and respiratory systems supply the oxygen demands of the body. Blood is oxygenated through the mechanisms of ventilation, perfusion, and transport of respiratory gases. Neural and chemical regulators control the rate and depth of respiration in response to changing tissue oxygen demands. The cardiovascular system provides the transport mechanisms to distribute oxygen to cells and tissues of the body.
Respiratory Physiology
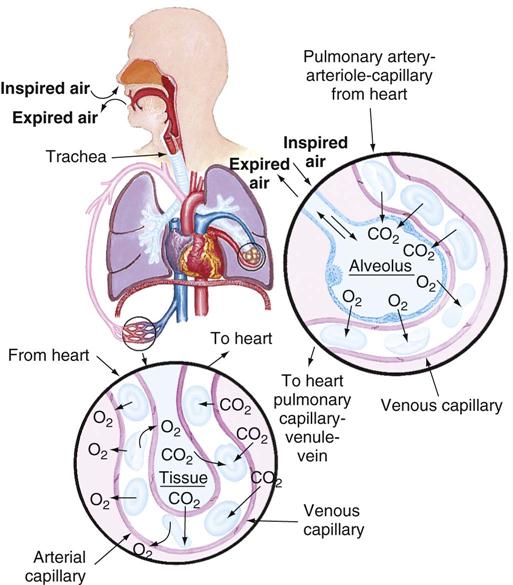
The exchange of respiratory gases occurs between the environment and the blood. Respiration is the exchange of oxygen and carbon dioxide during cellular metabolism. The airways of the lung transfer oxygen from the atmosphere to the alveoli, where the oxygen is exchanged for carbon dioxide. Through the alveolar capillary membrane, oxygen transfers to the blood, and carbon dioxide transfers from the blood to the alveoli. There are three steps in the process of oxygenation: ventilation, perfusion, and diffusion.
Structure and Function.
Conditions or diseases that change the structure and function of the pulmonary system alter respiration. The respiratory muscles, pleural space, lungs, and alveoli (Fig. 40-1) are essential for ventilation, perfusion, and exchange of respiratory gases. Gases move into and out of the lungs through pressure changes. Intrapleural pressure is negative, or less than atmospheric pressure, which is 760 mm Hg at sea level. For air to flow into the lungs, intrapleural pressure becomes more negative, setting up a pressure gradient between the atmosphere and the alveoli. The diaphragm and external intercostal muscles contract to create a negative pleural pressure and increase the size of the thorax for inspiration. Relaxation of the diaphragm and contraction of the internal intercostal muscles allow air to escape from the lungs.
Ventilation is the process of moving gases into and out of the lungs. It requires coordination of the muscular and elastic properties of the lung and thorax. The major inspiratory muscle of respiration is the diaphragm. It is innervated by the phrenic nerve, which exits the spinal cord at the fourth cervical vertebra. Perfusion relates to the ability of the cardiovascular system to pump oxygenated blood to the tissues and return deoxygenated blood to the lungs. Finally, diffusion is responsible for moving the respiratory gases from one area to another by concentration gradients. For the exchange of respiratory gases to occur, the organs, nerves, and muscles of respiration need to be intact; and the central nervous system needs to be able to regulate the respiratory cycle.
Work of Breathing.
Work of breathing (WOB) is the effort required to expand and contract the lungs. In the healthy individual breathing is quiet and accomplished with minimal effort. The amount of energy expended on breathing depends on the rate and depth of breathing, the ease in which the lungs can be expanded (compliance), and airway resistance.
Inspiration is an active process, stimulated by chemical receptors in the aorta. Expiration is a passive process that depends on the elastic recoil properties of the lungs, requiring little or no muscle work. Surfactant is a chemical produced in the lungs to maintain the surface tension of the alveoli and keep them from collapsing. Patients with advanced chronic obstructive pulmonary disease (COPD) lose the elastic recoil of the lungs and thorax. As a result, the patient’s work of breathing increases. In addition, patients with certain pulmonary diseases have decreased surfactant production and sometimes develop atelectasis. Atelectasis is a collapse of the alveoli that prevents normal exchange of oxygen and carbon dioxide.
Accessory muscles of respiration can increase lung volume during inspiration. Patients with COPD, especially emphysema, frequently use these muscles to increase lung volume. Prolonged use of the accessory muscles does not promote effective ventilation and causes fatigue. During assessment observe for elevation of the patient’s clavicles during inspiration, which can indicate ventilatory fatigue, air hunger, or decreased lung expansion.
Compliance is the ability of the lungs to distend or expand in response to increased intraalveolar pressure. Compliance decreases in diseases such as pulmonary edema, interstitial and pleural fibrosis, and congenital or traumatic structural abnormalities such as kyphosis or fractured ribs.
Airway resistance is the increase in pressure that occurs as the diameter of the airways decreases from mouth/nose to alveoli. Any further decrease in airway diameter by bronchoconstriction can increase airway resistance. Diseases causing airway obstruction such as asthma and tracheal edema increase airway resistance. When airway resistance increases, the amount of oxygen delivered to the alveoli decreases.
Decreased lung compliance, increased airway resistance, and the increased use of accessory muscles increase the WOB, resulting in increased energy expenditure. Therefore the body increases its metabolic rate and the need for more oxygen. The need for elimination of carbon dioxide also increases. This sequence is a vicious cycle for a patient with impaired ventilation, causing further deterioration of respiratory status and the ability to oxygenate adequately.
Lung Volumes.
The normal lung values are determined by age, gender, and height. Tidal volume is the amount of air exhaled after normal inspiration. Residual volume is the amount of air left in the alveoli after a full expiration. Forced vital capacity is the maximum amount of air that can be removed from the lungs during forced expiration (McCance and Huether, 2010). Variations in tidal volume and other lung volumes are associated with alterations in patients’ health status or activity, such as pregnancy, exercise, obesity, or obstructive and restrictive conditions of the lungs.
Pulmonary Circulation.
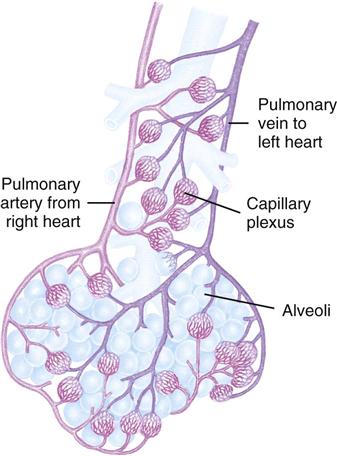
The primary function of pulmonary circulation is to move blood to and from the alveolar capillary membrane for gas exchange. Pulmonary circulation begins at the pulmonary artery, which receives poorly oxygenated mixed venous blood from the right ventricle. Blood flow through this system depends on the pumping ability of the right ventricle. The flow continues from the pulmonary artery through the pulmonary arterioles to the pulmonary capillaries, where blood comes in contact with the alveolar capillary membrane and the exchange of respiratory gases occurs. The oxygen-rich blood then circulates through the pulmonary venules and pulmonary veins, returning to the left atrium.
Respiratory Gas Exchange.
Diffusion is the process for the exchange of respiratory gases in the alveoli and the capillaries of the body tissues. Diffusion of respiratory gases occurs at the alveolar capillary membrane (Fig. 40-2). The thickness of the membrane affects the rate of diffusion. Increased thickness of the membrane impedes diffusion because gases take longer to transfer across the membrane. Patients with pulmonary edema, pulmonary infiltrates, or pulmonary effusion have a thickened membrane, resulting in slow diffusion, slow exchange of respiratory gases, and decreased delivery of oxygen to tissues. Chronic diseases (e.g., emphysema), acute diseases (e.g., pneumothorax), and surgical processes (e.g., lobectomy) often alter the amount of alveolar capillary membrane surface area.
Oxygen Transport.
The oxygen-transport system consists of the lungs and cardiovascular system. Delivery depends on the amount of oxygen entering the lungs (ventilation), blood flow to the lungs and tissues (perfusion), rate of diffusion, and oxygen-carrying capacity. Three things influence the capacity of the blood to carry oxygen: the amount of dissolved oxygen in the plasma, the amount of hemoglobin, and the tendency of hemoglobin to bind with oxygen. Hemoglobin, which is a carrier for oxygen and carbon dioxide, transports most oxygen (approximately 97%). The hemoglobin molecule combines with oxygen to form oxyhemoglobin. The formation of oxyhemoglobin is easily reversible, allowing hemoglobin and oxygen to dissociate (deoxyhemoglobin), which frees oxygen to enter tissues.
Carbon Dioxide Transport.
Carbon dioxide, a product of cellular metabolism, diffuses into red blood cells and is rapidly hydrated into carbonic acid (H2CO3). The carbonic acid then dissociates into hydrogen (H) and bicarbonate (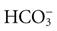 ) ions. Hemoglobin buffers the hydrogen ion, and the (
) ions. Hemoglobin buffers the hydrogen ion, and the (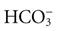 ) diffuses into the plasma (see Chapter 41). Reduced hemoglobin (deoxyhemoglobin) combines with carbon dioxide, and the venous blood transports the majority of carbon dioxide back to the lungs to be exhaled.
) diffuses into the plasma (see Chapter 41). Reduced hemoglobin (deoxyhemoglobin) combines with carbon dioxide, and the venous blood transports the majority of carbon dioxide back to the lungs to be exhaled.
Regulation of Respiration.
Regulation of respiration is necessary to ensure sufficient oxygen intake and carbon dioxide elimination to meet the demands of the body (e.g., during exercise, infection, or pregnancy). Neural and chemical regulators control the process of respiration. Neural regulation includes the central nervous system control of respiratory rate, depth, and rhythm. The cerebral cortex regulates the voluntary control of respiration by delivering impulses to the respiratory motor neurons by way of the spinal cord. Chemical regulation maintains the appropriate rate and depth of respirations based on changes in the carbon dioxide (CO2), oxygen (O2), and hydrogen ion (H+) concentration (pH) in the blood. Changes in chemical content of O2, CO2, and H (pH) stimulate the chemoreceptors located in the medulla, aortic body, and carotid body, which in turn stimulate neural regulators to adjust the rate and depth of ventilation to maintain normal arterial blood gas levels.
Cardiovascular Physiology
Cardiopulmonary physiology involves delivery of deoxygenated blood (blood high in carbon dioxide and low in oxygen) to the right side of the heart and then to the lungs, where it is oxygenated. Oxygenated blood (blood high in oxygen and low in carbon dioxide) then travels from the lungs to the left side of the heart and the tissues. The cardiac system delivers oxygen, nutrients, and other substances to the tissues and facilitates the removal of cellular metabolism waste products by way of blood flow through other body systems such as respiratory, digestive, and renal (McCance and Huether, 2010).
Structure and Function.
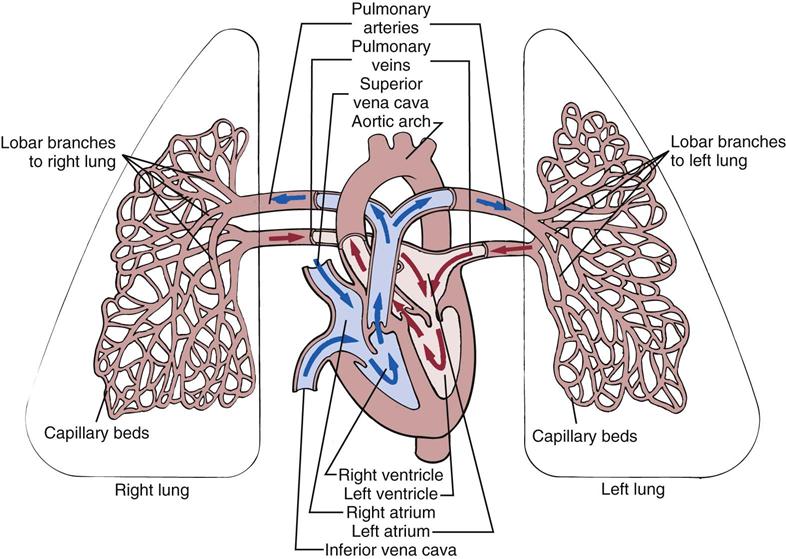
The right ventricle pumps deoxygenated blood through the pulmonary circulation (Fig. 40-3). The left ventricle pumps oxygenated blood through the systemic circulation. As blood passes through the circulatory system, there is an exchange of respiratory gases, nutrients, and waste products between the blood and the tissues.
Myocardial Pump.
The pumping action of the heart is essential to oxygen delivery. There are four cardiac chambers, two atria and two ventricles. The ventricles fill with blood during diastole and empty during systole. The volume of blood ejected from the ventricles during systole is the stroke volume. Hemorrhage and dehydration cause a decrease in circulating blood volume and a decrease in stroke volume.
Myocardial fibers have contractile properties that allow them to stretch during filling. In a healthy heart this stretch is proportionally related to the strength of contraction. As the myocardium stretches, the strength of the subsequent contraction increases; this is known as the Frank-Starling (Starling’s) law of the heart. In the diseased heart (cardiomyopathy or myocardial infarction [MI]) Starling’s law does not apply because the increased stretch of the myocardium is beyond the physiological limits of the heart. The subsequent contractile response results in insufficient stroke volume, and blood begins to “back up” in the pulmonary (left heart failure) or systemic (right heart failure) circulation.
Myocardial Blood Flow.
To maintain adequate blood flow to the pulmonary and systemic circulation, myocardial blood flow must supply sufficient oxygen and nutrients to the myocardium itself. Blood flow through the heart is unidirectional. The four heart valves ensure this forward blood flow (see Fig. 40-3). During ventricular diastole the atrioventricular (mitral and tricuspid) valves open, and blood flows from the higher-pressure atria into the relaxed ventricles. As systole begins, ventricular pressure rises and closes the mitral and tricuspid valves. Valve closure causes the first heart sound (S1).
During the systolic phase the semilunar (aortic and pulmonic) valves open, and blood flows from the ventricles into the aorta and pulmonary artery. The mitral and tricuspid valves stay closed during systole so all of the blood is moved forward into the pulmonary artery and aorta. As the ventricles empty, the ventricular pressures decrease, allowing closure of the aortic and pulmonic valves, which causes the second heart sound (S2). Some patients with valvular disease have backflow or regurgitation of blood through the incompetent valve, causing a murmur that you can hear on auscultation (see Chapter 30).
Coronary Artery Circulation.
The coronary circulation is the branch of the systemic circulation that supplies the myocardium with oxygen and nutrients and removes waste. The coronary arteries fill during ventricular diastole (McCance and Huether, 2010). The left coronary artery has the most abundant blood supply and feeds the more muscular left ventricular myocardium, which does most of the work of the heart.
Systemic Circulation.
The arteries of the systemic circulation deliver nutrients and oxygen to tissues, and the veins remove waste from tissues. Oxygenated blood flows from the left ventricle through the aorta and into large systemic arteries. These arteries branch into smaller arteries; then arterioles; and finally the smallest vessels, the capillaries. The exchange of respiratory gases occurs at the capillary level, where the tissues are oxygenated. The waste products exit the capillary network through venules that join to form veins. These veins become larger and form the vena cava, which carry deoxygenated blood to the right side of the heart, where it then returns to the pulmonary circulation.
Blood Flow Regulation.
The amount of blood ejected from the left ventricle each minute is the cardiac output. The normal cardiac output is 4 to 6 L/min in the healthy adult at rest. The circulating volume of blood changes according to the oxygen and metabolic needs of the body. For example, cardiac output increases during exercise, pregnancy, and fever but decreases during sleep. The following formula represents cardiac output:
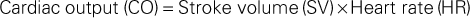
The amount of blood in the left ventricle at the end of diastole (preload), the resistance to left ventricular ejection (afterload), and myocardial contractility all affect stroke volume.
Preload is the end-diastolic volume. The ventricles stretch when filling with blood. The more stretch on the ventricular muscle, the greater the contraction and the greater the stroke volume (Starling’s law). In clinical situations, medical treatment can alter the preload and subsequent stroke volumes by changing the amount of circulating blood volume. For example, during treatment of a patient who is hemorrhaging, increased fluid therapy and replacement of blood increase circulating volume, thus increasing the preload and stroke volume, which increases cardiac output. If volume is not replaced, preload, stroke volume and the subsequent cardiac output decreases.
Afterload is the resistance to left ventricular ejection. The heart works harder to overcome the resistance so blood can be fully ejected from the left ventricle. The diastolic aortic pressure is a good clinical measure of afterload. In hypertension the afterload increases, making cardiac workload also increase.
Myocardial contractility also affects stroke volume and cardiac output. Poor ventricular contraction decreases the amount of blood ejected. Injury to the myocardial muscle such as an acute MI causes a decrease in myocardial contractility. The myocardium of the older adult is stiffer with a slower ventricular filling rate and prolonged contraction time (Linton and Lach, 2007).
Heart rate affects blood flow because of the relationship between rate and diastolic filling time. With a sustained heart rate greater than 160 beats/min, diastolic filling time decreases, decreasing stroke volume and cardiac output. The heart rate of the older adult is slow to increase under stress, but studies have found that this may be caused more by lack of conditioning than age. Exercise is beneficial in maintaining function at any age (Linton and Lach, 2007).
Conduction System.
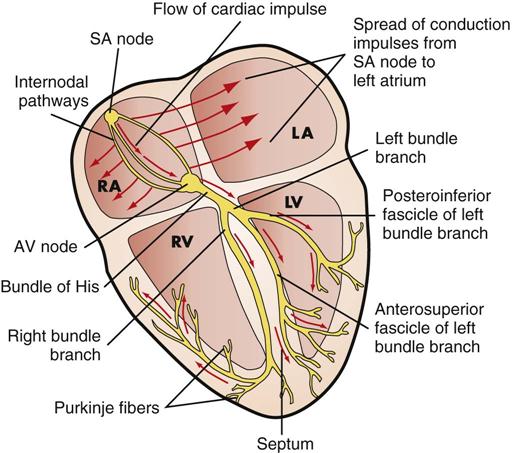
The rhythmic relaxation and contraction of the atria and ventricles depend on continuous, organized transmission of electrical impulses. The cardiac conduction system generates and transmits these impulses (Fig. 40-4).
The conduction system of the heart generates the impulses needed to initiate the electrical chain of events for a normal heartbeat. The autonomic nervous system influences the rate of impulse generation and the speed of transmission through the conductive pathway and the strength of atrial and ventricular contractions. Sympathetic and parasympathetic nerve fibers innervate all parts of the atria and ventricles and the sinoatrial (SA) and atrioventricular (AV) nodes. Sympathetic fibers increase the rate of impulse generation and speed of transmission. The parasympathetic fibers originating from the vagus nerve decrease the rate.
The conduction system originates with the SA node, the “pacemaker” of the heart. The SA node is in the right atrium next to the entrance of the superior vena cava. Impulses are initiated at the SA node at an intrinsic rate of 75 cardiac action potentials per minute in an adult at rest (McCance and Huether, 2010).
The electrical impulses are transmitted through the atria along intraatrial pathways to the AV node. The AV node mediates impulses between the atria and the ventricles. It assists atrial emptying by delaying the impulse before transmitting it through the bundle of His and the ventricular Purkinje network.
An electrocardiogram (ECG) reflects the electrical activity of the conduction system. An ECG monitors the regularity and path of the electrical impulse through the conduction system; however, it does not reflect the muscular work of the heart. The normal sequence on the ECG is called the normal sinus rhythm (NSR) (Fig. 40-5).
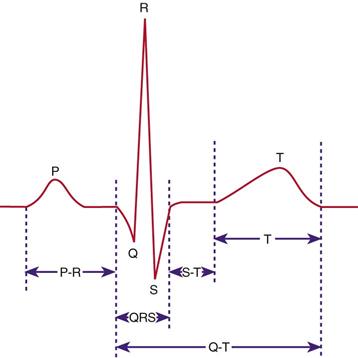
NSR implies that the impulse originates at the SA node and follows the normal sequence through the conduction system. The P wave represents the electrical conduction through both atria. Atrial contraction follows the P wave. The PR interval represents the impulse travel time from the SA node through the AV node, through the bundle of His, and to the Purkinje fibers. The normal length for the PR interval is 0.12 to 0.2 second. An increase in the time greater than 0.2 second indicates a block in the impulse transmission through the AV node; whereas a decrease, less than 0.12 second, indicates the initiation of the electrical impulse from a source other than the SA node.
The QRS complex indicates that the electrical impulse traveled through the ventricles. Normal QRS duration is 0.06 to 0.1 second. An increase in QRS duration indicates a delay in conduction time through the ventricles. Ventricular contraction usually follows the QRS complex.
The QT interval represents the time needed for ventricular depolarization and repolarization. The normal QT interval is 0.12 to 0.42 second. This interval varies inversely with changes in heart rate (McCance and Huether, 2010). Changes in electrolyte values such as hypocalcemia or therapy with drugs such as disopyramide (Norpace) or amiodarone (Cordarone) increase the QT interval. Shortening of the QT interval occurs with digitalis therapy, hyperkalemia, and hypercalcemia.
Factors Affecting Oxygenation
Four factors influence adequacy of circulation, ventilation, perfusion, and transport of respiratory gases to the tissues: (1) physiological, (2) developmental, (3) lifestyle, and (4) environmental. The physiological factors are discussed here, and the others are discussed in the Nursing Knowledge Base section that follows.
Physiological Factors.
Any condition affecting cardiopulmonary functioning directly affects the ability of the body to meet oxygen demands. Respiratory disorders include hyperventilation, hypoventilation, and hypoxia. Cardiac disorders include disturbances in conduction, impaired valvular function, myocardial hypoxia, cardiomyopathic conditions, and peripheral tissue hypoxia. Other physiological processes affecting a patient’s oxygenation include alterations affecting the oxygen-carrying capacity of blood, decreased inspired oxygen concentration, increases in the metabolic demand of the body, and alterations affecting chest wall movement caused by musculoskeletal abnormalities or neuromuscular alterations.
Decreased Oxygen-Carrying Capacity.
Hemoglobin carries the majority of oxygen to tissues. Anemia and inhalation of toxic substances decrease the oxygen-carrying capacity of blood by reducing the amount of available hemoglobin to transport oxygen. Anemia (i.e., a lower-than-normal hemoglobin level) is a result of decreased hemoglobin production, increased red blood cell destruction, and/or blood loss. Patients have fatigue, decreased activity tolerance, increased breathlessness, increased heart rate, and pallor (especially seen in the conjunctiva of the eye). Oxygenation decreases as a secondary effect with anemia. The physiological response to chronic hypoxemia is the development of increased red blood cells (polycythemia). This is the adaptive response of the body to increase the amount of hemoglobin and the available oxygen-binding sites.
Carbon monoxide (CO) is the most common toxic inhalant decreasing the oxygen-carrying capacity of blood. In CO toxicity hemoglobin strongly binds with CO, creating a functional anemia. Because of the strength of the bond, CO does not easily dissociate from hemoglobin, making hemoglobin unavailable for oxygen transport.
Hypovolemia.
Conditions such as shock and severe dehydration cause extracellular fluid loss and reduced circulating blood volume, or hypovolemia. Decreased circulating blood volume results in hypoxia to body tissues. With significant fluid loss, the body tries to adapt by peripheral vasoconstriction and increasing the heart rate to increase the volume of blood returned to the heart, thus increasing the cardiac output.
Decreased Inspired Oxygen Concentration.
With the decline of the concentration of inspired oxygen, the oxygen-carrying capacity of the blood decreases. Decreases in the fraction of inspired oxygen concentration (FiO2) are caused by upper or lower airway obstruction, which limits delivery of inspired oxygen to alveoli; decreased environmental oxygen (at high altitudes); or hypoventilation (occurs in drug overdoses).
Increased Metabolic Rate.
Increased metabolic activity increases oxygen demand. The level of oxygenation declines when body systems are unable to meet this demand. An increased metabolic rate is normal in pregnancy, wound healing, and exercise because the body is using energy or building tissue. Most people are able to meet the increased oxygen demand and do not display signs of oxygen deprivation. Fever increases the need of tissues for oxygen; as a result carbon dioxide production increases. When fever persists, the metabolic rate remains high, and the body begins to break down protein stores. This causes muscle wasting and decreased muscle mass, including respiratory muscles such as the diaphragm and intercostal muscles.
The body attempts to adapt to the increased carbon dioxide levels by increasing the rate and depth of respiration. The patient’s WOB increases, and the patient eventually displays signs and symptoms of hypoxemia. Patients with pulmonary diseases are at greater risk for hypoxemia.
Conditions Affecting Chest Wall Movement.
Any condition reducing chest wall movement results in decreased ventilation. If the diaphragm does not fully descend with breathing, the volume of inspired air decreases, delivering less oxygen to the alveoli and tissues.
Pregnancy.
As the fetus grows during pregnancy, the enlarging uterus pushes abdominal contents upward against the diaphragm. In the last trimester of pregnancy, the inspiratory capacity declines, resulting in dyspnea on exertion and increased fatigue.
Obesity.
Patients who are morbidly obese have reduced lung volumes from the heavy lower thorax and abdomen, particularly when in the recumbent and supine positions. Many morbidly obese patients suffer from obstructive sleep apnea. Morbidly obese patients have a reduction in lung and chest wall compliance as a result of encroachment of the abdomen into the chest, increased WOB, and decreased lung volumes. In some patients an obesity-hypoventilation syndrome develops in which oxygenation is decreased and carbon dioxide is retained. The obese patient is also susceptible to atelectasis or pneumonia after surgery because the lungs do not expand fully and the lower lobes retain pulmonary secretions.
Musculoskeletal Abnormalities.
Musculoskeletal impairments in the thoracic region reduce oxygenation. Such impairments result from abnormal structural configurations, trauma, muscular diseases, and diseases of the central nervous system. Abnormal structural configurations impairing oxygenation include those affecting the rib cage such as pectus excavatum and the vertebral column such as kyphosis, lordosis, or scoliosis.
Trauma.
Flail chest is a condition in which multiple rib fractures cause instability in part of the chest wall. The unstable chest wall allows the lung underlying the injured area to contract on inspiration and bulge on expiration, resulting in hypoxia. Patients with thoracic or upper abdominal surgical incisions use shallow respirations to avoid pain, which also decreases chest wall movement. Opioids used to treat pain depress the respiratory center, further decreasing respiratory rate and chest wall expansion.
Neuromuscular Diseases.
Neuromuscular diseases affect tissue oxygenation by decreasing the patient’s ability to expand and contract the chest wall. Ventilation is impaired, resulting in atelectasis, hypercapnia, and hypoxemia. Examples of conditions causing hypoventilation include myasthenia gravis, Guillain-Barré syndrome, and poliomyelitis.
Central Nervous System Alterations.
Diseases or trauma of the medulla oblongata and/or spinal cord result in impaired respiration. When the medulla oblongata is affected, neural regulation of respiration is impaired, and abnormal breathing patterns develop. Cervical trauma at C3 to C5 usually results in paralysis of the phrenic nerve. When the phrenic nerve is damaged, the diaphragm does not descend properly, thus reducing inspiratory lung volumes and causing hypoxemia. Spinal cord trauma below the C5 vertebra usually leaves the phrenic nerve intact but damages nerves that innervate the intercostal muscles, preventing anteroposterior chest expansion.
Influences of Chronic Disease.
Oxygenation decreases as a direct consequence of chronic lung disease. Changes in the anteroposterior diameter of the chest wall (barrel chest) occur because of overuse of accessory muscles and air trapping in emphysema. The diaphragm is flattened, and the lung fields are overdistended, resulting in varying degrees of hypoxemia and/or hypercapnia (McCance and Huether, 2010).
Alterations in Respiratory Functioning
Illnesses and conditions affecting ventilation or oxygen transport cause alterations in respiratory functioning. The three primary alterations are hypoventilation, hyperventilation, and hypoxia.
The goal of ventilation is to produce a normal arterial carbon dioxide tension (PaCO2) between 35 and 45 mm Hg and a normal arterial oxygen tension (PaO2) between 80 and 100 mm Hg. Hypoventilation and hyperventilation are often determined by arterial blood gas analysis (McCance and Huether, 2010). Hypoxemia refers to a decrease in the amount of arterial oxygen. Nurses monitor arterial oxygen saturation (SpO2) using a noninvasive oxygen saturation monitor pulse oximeter. Normally SpO2 is greater than or equal to 95% (see Chapter 41).
Hypoventilation.
Hypoventilation occurs when alveolar ventilation is inadequate to meet the oxygen demand of the body or eliminate sufficient carbon dioxide. As alveolar ventilation decreases, the body retains carbon dioxide. For example, atelectasis, a collapse of the alveoli, prevents normal exchange of oxygen and carbon dioxide. As more alveoli collapse, less of the lung is ventilated, and hypoventilation occurs.
In patients with COPD, the administration of excessive oxygen results in hypoventilation. These patients have adapted to a high carbon dioxide level so their carbon dioxide–sensitive chemoreceptors are essentially not functioning. Their peripheral chemoreceptors of the aortic arch and carotid bodies are primarily sensitive to lower oxygen levels, causing increased ventilation. Because the stimulus to breathe is a decreased arterial oxygen (PaO2) level, administration of oxygen greater than 24% to 28% (1 to 3 L/min) prevents the PaO2 from falling to a level (60 mm Hg) that stimulates the peripheral receptors, thus destroying the stimulus to breathe (McCance and Huether, 2010). The resulting hypoventilation causes excessive retention of carbon dioxide, which can lead to respiratory acidosis and respiratory arrest.
Signs and symptoms of hypoventilation include mental status changes, dysrhythmias, and potential cardiac arrest. If untreated, the patient’s status rapidly declines, leading to convulsions, unconsciousness, and death.
Hyperventilation.
Hyperventilation is a state of ventilation in which the lungs remove carbon dioxide faster than it is produced by cellular metabolism. Severe anxiety, infection, drugs, or an acid-base imbalance induces hyperventilation. Acute anxiety leads to hyperventilation and exhalation of excessive amounts of carbon dioxide. Increased body temperature (fever) increases the metabolic rate, thereby increasing carbon dioxide production. The increased carbon dioxide level stimulates an increase in the patient’s rate and depth of respiration, causing hyperventilation.
Hyperventilation is sometimes chemically induced. Salicylate (aspirin) poisoning and amphetamine use result in excess carbon dioxide production, stimulating the respiratory center to compensate by increasing the rate and depth of respiration. It also occurs as the body tries to compensate for metabolic acidosis. For example, the patient with diabetes in ketoacidosis produces large amounts of metabolic acids. The respiratory system tries to correct the acid-base balance by overbreathing. Ventilation increases to reduce the amount of carbon dioxide available to form carbonic acid (see Chapter 41). This can also result in the patient developing respiratory alkalosis. Signs and symptoms of hyperventilation include rapid respirations, sighing breaths, numbness and tingling of hands/feet, light-headedness, and loss of consciousness (Ackley and Ladwig, 2011).
Hypoxia.
Hypoxia is inadequate tissue oxygenation at the cellular level. It results from a deficiency in oxygen delivery or oxygen use at the cellular level. It is a life-threatening condition. Untreated it produces possibly fatal cardiac dysrhythmias.
Causes of hypoxia include (1) a decreased hemoglobin level and lowered oxygen-carrying capacity of the blood; (2) a diminished concentration of inspired oxygen, which occurs at high altitudes; (3) the inability of the tissues to extract oxygen from the blood, as with cyanide poisoning; (4) decreased diffusion of oxygen from the alveoli to the blood, as in pneumonia; (5) poor tissue perfusion with oxygenated blood, as with shock; and (6) impaired ventilation, as with multiple rib fractures or chest trauma.
The clinical signs and symptoms of hypoxia include apprehension, restlessness, inability to concentrate, decreased level of consciousness, dizziness, and behavioral changes. The patient with hypoxia is unable to lie flat and appears both fatigued and agitated. Vital sign changes include an increased pulse rate and rate and depth of respiration. During early stages of hypoxia the blood pressure is elevated unless the condition is caused by shock. As the hypoxia worsens, the respiratory rate declines as a result of respiratory muscle fatigue.
Cyanosis, blue discoloration of the skin and mucous membranes caused by the presence of desaturated hemoglobin in capillaries, is a late sign of hypoxia. The presence or absence of cyanosis is not a reliable measure of oxygen status. Central cyanosis, observed in the tongue, soft palate, and conjunctiva of the eye where blood flow is high, indicates hypoxemia. Peripheral cyanosis, seen in the extremities, nail beds, and earlobes, is often a result of vasoconstriction and stagnant blood flow.
Alterations in Cardiac Functioning
Illnesses and conditions affecting cardiac rhythm, strength of contraction, blood flow through the heart or to the heart muscle, and decreased peripheral circulation cause alterations in cardiac functioning. Older adults experience alterations in cardiac function as a result of calcification of the conduction pathways, thicker and stiffer heart valves caused by lipid accumulation and fibrosis, and a decrease in the number of pacemaker cells in the SA node (Linton and Lach, 2007; Meiner, 2011).
Disturbances in Conduction.
Electrical impulses that do not originate from the SA node cause conduction disturbances. These rhythm disturbances are called dysrhythmias, meaning a deviation from the normal sinus heart rhythm. Dysrhythmias occur as a primary conduction disturbance such as in response to ischemia; valvular abnormality; anxiety; drug toxicity; caffeine, alcohol, or tobacco use; or as a complication of acid-base or electrolyte imbalance (see Chapter 41).
Dysrhythmias are classified by cardiac response and site of impulse origin. Cardiac response is tachycardia (greater than 100 beats/min), bradycardia (less than 60 beats/min), a premature (early) beat, or a blocked (delayed or absent) beat. Tachydysrhythmias and bradydysrhythmias lower cardiac output and blood pressure. Tachydysrhythmias reduce cardiac output by decreasing diastolic filling time. Bradydysrhythmias lower cardiac output because of the decreased heart rate.
Atrial fibrillation is a common dysrhythmia frequently seen in older adults. The electrical impulse in the atria is chaotic and originates from multiple sites. The rhythm is irregular because of the multiple pacemaker sites and the unpredictable conduction to the ventricles. The QRS complex is normal; however, it occurs at irregular intervals. Atrial fibrillation is often described as an irregularly irregular rhythm.
Abnormal impulses originating above the ventricles are supraventricular dysrhythmias. The abnormality on the waveform is the configuration and placement of the P wave. Ventricular conduction usually remains normal, and there is a normal QRS complex.
Paroxysmal supraventricular tachycardia is a sudden, rapid onset of tachycardia originating above the AV node. It often begins and ends spontaneously. Sometimes excitement, fatigue, caffeine, smoking, or alcohol use precipitates paroxysmal supraventricular tachycardia.
Ventricular dysrhythmias represent an ectopic site of impulse formation within the ventricles. It is ectopic in that the impulse originates in the ventricle, not the SA node. The configuration of the QRS complex is usually widened and bizarre. P waves are not always present; often they are buried in the QRS complex. Ventricular tachycardia and ventricular fibrillation are life-threatening rhythms that require immediate intervention. Ventricular tachycardia is a life-threatening dysrhythmia because of the decreased cardiac output and the potential to deteriorate into ventricular fibrillation or sudden cardiac death (AHA, 2010b).
Altered Cardiac Output.
Failure of the myocardium to eject sufficient volume to the systemic and pulmonary circulations occurs in heart failure. Primary coronary artery disease, cardiomyopathy, valvular disorders, and pulmonary disease lead to myocardial pump failure.
Left-Sided Heart Failure.
Left-sided heart failure is an abnormal condition characterized by decreased functioning of the left ventricle. If left ventricular failure is significant, the amount of blood ejected from the left ventricle drops greatly, resulting in decreased cardiac output. Signs and symptoms include fatigue, breathlessness, dizziness, and confusion as a result of tissue hypoxia from the diminished cardiac output. As the left ventricle continues to fail, blood begins to pool in the pulmonary circulation, causing pulmonary congestion. Clinical findings include crackles in the bases of the lungs on auscultation, hypoxia, shortness of breath on exertion, cough, and paroxysmal nocturnal dyspnea.
Right-Sided Heart Failure.
Right-sided heart failure results from impaired functioning of the right ventricle. It more commonly results from pulmonary disease or as a result of long-term left-sided failure. The primary pathological factor in right-sided failure is elevated pulmonary vascular resistance (PVR). As the PVR continues to rise, the right ventricle works harder, and the oxygen demand of the heart increases. As the failure continues, the amount of blood ejected from the right ventricle declines, and blood begins to “back up” in the systemic circulation. Clinically the patient has weight gain, distended neck veins, hepatomegaly and splenomegaly, and dependent peripheral edema.
Impaired Valvular Function.
Valvular heart disease is an acquired or congenital disorder of a cardiac valve that causes either hardening (stenosis) or impaired closure (regurgitation) of the valves. When stenosis occurs, the flow of blood through the valves is obstructed. For example, when stenosis occurs in the semilunar valves (aortic and pulmonic valves), the adjacent ventricles have to work harder to move the ventricular blood volume beyond the stenotic valve. Over time the stenosis causes the ventricle to hypertrophy (enlarge); and, if the condition is untreated, left- or right-sided heart failure occurs. When regurgitation occurs, there is a backflow of blood into an adjacent chamber. For example, in mitral regurgitation the mitral leaflets do not close completely. When the ventricles contract, blood escapes back into the atria, causing a murmur, or “whooshing” sound (see Chapter 30).
Myocardial Ischemia.
Myocardial ischemia results when the supply of blood to the myocardium from the coronary arteries is insufficient to meet myocardial oxygen demands. Two common outcomes of this ischemia are angina pectoris and MI.
Angina.
Angina pectoris is a transient imbalance between myocardial oxygen supply and demand. The condition results in chest pain that is aching, sharp, tingling, or burning or that feels like pressure. Typically chest pain is left sided or substernal and often radiates to the left or both arms, the jaw, neck, and back. In some patients angina pain does not radiate. It usually lasts from 3 to 5 minutes (McCance and Huether, 2010). Patients report that it is often precipitated by activities that increase myocardial oxygen demand (e.g., eating heavy meals, exercise, or stress). It is usually relieved with rest and coronary vasodilators, the most common being a nitroglycerin preparation.
Myocardial Infarction.
Myocardial infarction (MI) or acute coronary syndrome (ACS) results from sudden decreases in coronary blood flow or an increase in myocardial oxygen demand without adequate coronary perfusion. Infarction occurs because ischemia is not reversed. Cellular death occurs after 20 minutes of myocardial ischemia (McCance and Huether, 2010).
Chest pain associated with MI in men is usually described as crushing, squeezing, or stabbing. The pain is often in the left chest and sternal area; may be felt in the back; and radiates down the left arm to the neck, jaws, teeth, epigastric area, and back. It occurs at rest or exertion and lasts more than 20 minutes. Rest, position change, or sublingual nitroglycerin administration does not relieve the pain.
There is a significant difference between men and women in relation to coronary artery disease. As women get older, their risk of heart disease begins to rise (AHA, 2010a). Women on average have greater blood cholesterol and triglyceride levels than men. Obesity in women is more prevalent, which also increases risk for diabetes and cardiac disease (Shaw et al., 2009). Women’s symptoms differ from those of men. The most common initial symptom in women is angina, but they also present with atypical symptoms such as fatigue, indigestion, shortness of breath, and back or jaw pain. Women have twice the risk of dying within the first year after a heart attack than men.
Nursing Knowledge Base
Factors Influencing Oxygenation
In addition to physiological factors, multiple developmental, lifestyle, and environmental factors affect patients’ oxygenation status. It is important to recognize these as possible risks or factors that impact their health care goals.
Developmental Factors.
The developmental stage of a patient and the normal aging process affect tissue oxygenation.
Infants and Toddlers.
Infants and toddlers are at risk for upper respiratory tract infections as a result of frequent exposure to other children, an immature immune system, and exposure to secondhand smoke. In addition, during the teething process some infants develop nasal congestion, which encourages bacterial growth and increases the potential for respiratory tract infection. Upper respiratory tract infections are usually not dangerous, and infants or toddlers recover with little difficulty.
School-Age Children and Adolescents.
School-age children and adolescents are exposed to respiratory infections and respiratory risk factors such as cigarette smoking or secondhand smoke. A healthy child usually does not have adverse pulmonary effects from respiratory infections. The American Lung Association (2008) reported a study showing that cigarette smoking in college students (19.2%) had declined in 2006 compared to those smoking in 1999 (30.6%). Although this is still higher than the national goal set by the U.S. Department of Health and Human Services (12%), there is hope that the decline will continue. The biggest risk factor for those still smoking in college was if they started smoking in high school (American Lung Association, 2008). A person who starts smoking in adolescence and continues to smoke into middle age has an increased risk for cardiopulmonary disease and lung cancer.
Young and Middle-Age Adults.
Young and middle-age adults are exposed to multiple cardiopulmonary risk factors: an unhealthy diet, lack of exercise, stress, over-the-counter and prescription drugs not used as intended, illegal substances, and smoking. Reducing these modifiable factors decreases a patient’s risk for cardiac or pulmonary diseases. This is also the time when individuals establish lifelong habits and lifestyles. In 2007 20.6% of adults were smokers (LungUSA, 2010). It is important to help your patients make good choices and informed decisions about their health care practices. The increased cost of cigarettes plus the state smoke-free air policies and laws that reduce smoking in public places have proven to be helpful in smoking cessation (American Lung Association, 2008).
Older Adults.
The cardiac and respiratory systems undergo changes throughout the aging process (Box 40-1). The changes are associated with calcification of the heart valves, SA node, and costal cartilages. The arterial system develops atherosclerotic plaques.
Osteoporosis leads to changes in the size and shape of the thorax. The trachea and large bronchi become enlarged from calcification of the airways. The alveoli enlarge, decreasing the surface area available for gas exchange. The number of functional cilia is reduced, causing a decrease in the effectiveness of the cough mechanism, putting the older adult at increased risk for respiratory infections (Meiner, 2011).
Lifestyle Factors.
Lifestyle modifications are difficult for patients because they often have to change an enjoyable habit such as cigarette smoking or eating certain foods. Risk-factor modification is important and includes smoking cessation, weight reduction, a low-cholesterol and low-sodium diet, management of hypertension, and moderate exercise (see Chapter 6). Although it is difficult to change long-term behavior, helping patients acquire healthy behaviors reduces the risk for or slows or halts the progression of cardiopulmonary diseases (Meiner, 2011).
Nutrition.
Nutrition affects cardiopulmonary function in several ways. Severe obesity decreases lung expansion, and increased body weight increases tissue oxygen demands. The malnourished patient experiences respiratory muscle wasting, resulting in decreased muscle strength and respiratory excursion. Cough efficiency is reduced secondary to respiratory muscle weakness, putting the patient at risk for retention of pulmonary secretions.
Patients who are morbidly obese and/or malnourished are at risk for anemia. Diets high in carbohydrates play a role in increasing the carbon dioxide load for patients with carbon dioxide retention. As carbohydrates are metabolized, an increased load of carbon dioxide is created and excreted via the lungs.
Dietary practices also influence the prevalence of cardiovascular diseases. Cardioprotective nutrition includes diets rich in fiber; whole grains; fresh fruits and vegetables; nuts; antioxidants; lean meats, fish, and chicken; and omega-3 fatty acids. The latest update by the Joint National Committee (JNC, 2003) recommended that dietary restriction of sodium is beneficial in reducing antihypertensive medication requirements; in some cases it causes left ventricular hypertrophy to regress. Diets high in potassium prevent hypertension and help improve control in patients with hypertension. A 2000-calorie diet of fruits; vegetables; and low-fat dairy foods that are high in fiber, potassium, calcium, and magnesium and low in saturated and total fat helps prevent and reduce the effects of hypertension.
Exercise.
Exercise increases the metabolic activity and oxygen demand of the body. The rate and depth of respiration increase, enabling the person to inhale more oxygen and exhale excess carbon dioxide. A physical exercise program has many benefits (see Chapter 38). People who exercise for 30 to 60 minutes daily have a lower pulse rate and blood pressure, decreased cholesterol level, increased blood flow, and greater oxygen extraction by working muscles. Fully conditioned people increase oxygen consumption by 10% to 20% because of increased cardiac output and increased efficiency of the myocardial muscle (JNC, 2003).
Smoking.
Cigarette smoking and secondhand smoke are associated with a number of diseases, including heart disease, COPD, and lung cancer. Cigarette smoking worsens peripheral vascular and coronary artery diseases (McCance and Huether, 2010). Inhaled nicotine causes vasoconstriction of peripheral and coronary blood vessels, increasing blood pressure and decreasing blood flow to peripheral vessels.
Women who take birth control pills and smoke cigarettes have an increased risk for thrombophlebitis and pulmonary emboli. Smoking during pregnancy can result in low-birth-weight babies, preterm delivery, and babies with reduced lung function (LungUSA, 2010). Even exposure to secondhand smoke can be a risk for low-birth-weight babies, preterm delivery, and miscarriages (ACS, 2010).
The risk of lung cancer is 10 times greater for a person who smokes than for a nonsmoker. In the United States the use of tobacco accounts for 30% of all cancer deaths. This includes 87% of the deaths from lung cancer and cancer of the larynx, mouth, pharynx, esophagus, and bladder. Smoking has been linked to the development of other cancers, including kidney, cervix, and leukemia (ACS, 2010). Nicotine patches, gum, and lozenges are available over the counter, and nicotine nasal spray and inhalers can be obtained by prescription. Prescription drugs such as bupropion (Zyban) and varenicline (Chantix) are also available to help people quit smoking (LungUSA, 2010).
Exposure to environmental tobacco smoke (secondhand smoke) increases the risk of lung cancer and cardiovascular disease in the nonsmoker. Children with parents who smoke have a higher incidence of asthma, pneumonia, and ear infections. Babies exposed to secondhand smoke are at higher risk for sudden infant death syndrome (ACS, 2010).
Substance Abuse.
Excessive use of alcohol and other drugs impairs tissue oxygenation in two ways. First, the person who chronically abuses substances often has a poor nutritional intake. With the resultant decrease in intake of iron-rich foods, hemoglobin production declines. Second, excessive use of alcohol and certain other drugs depresses the respiratory center, reducing the rate and depth of respiration and the amount of inhaled oxygen. Substance abuse by either smoking or inhaling substances such as crack cocaine or fumes from paint or glue cans causes direct injury to lung tissue that leads to permanent lung damage. The report on inhalant abuse (huffing) by teenagers to get a euphoric effect includes use of a wide variety of substances such as paint thinner, nail polish remover, glue, spray paint, nitrous oxide, and other common household products. Sudden death can occur from cardiac arrhythmias; or chronic abuse can cause damage to heart, lungs, and kidneys (Stoppler, 2005).
Stress.
A continuous state of stress or severe anxiety increases the metabolic rate and oxygen demand of the body. The body responds to anxiety and other stresses with an increased rate and depth of respiration. Most people adapt; but some, particularly those with chronic illnesses or acute life-threatening illnesses such as an MI, cannot tolerate the oxygen demands associated with anxiety (see Chapter 37).
Environmental Factors.
The environment also influences oxygenation. The incidence of pulmonary disease is higher in smoggy, urban areas than in rural areas. In addition, a patient’s workplace sometimes increases the risk for pulmonary disease. Occupational pollutants include asbestos, talcum powder, dust, and airborne fibers. For example, farm workers in dry regions of the southwestern United States are at risk for coccidioidomycosis, a fungal disease caused by inhalation of spores of the airborne bacterium Coccidioides immitis. Asbestosis is an occupational lung disease that develops after exposure to asbestos. The lung with asbestosis often has diffuse interstitial fibrosis, creating a restrictive lung disease. Patients exposed to asbestos are at risk for developing lung cancer, and this risk increases with exposure to tobacco smoke.
Critical Thinking
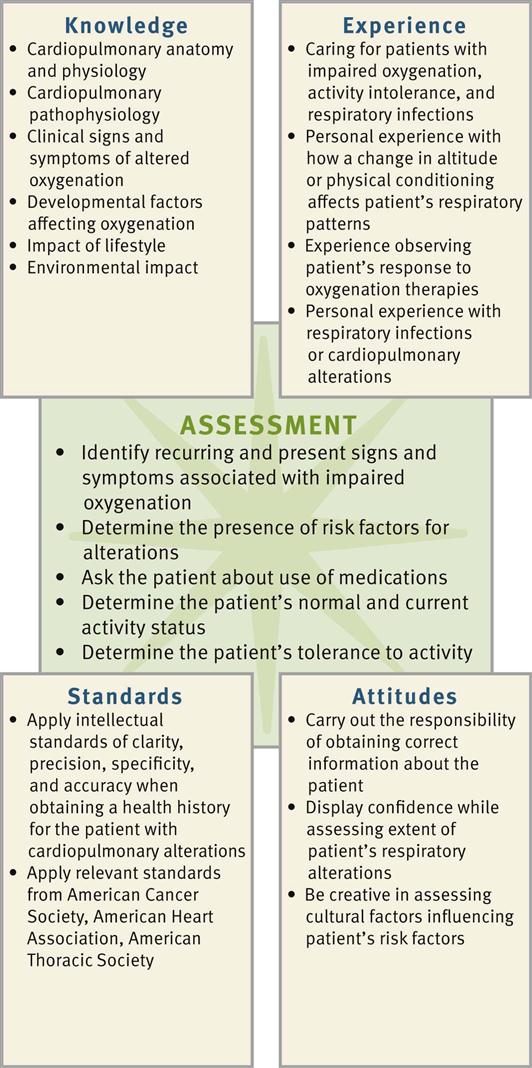
Successful critical thinking requires a synthesis of knowledge, experience, information gathered from patients, critical thinking attitudes, and intellectual and professional standards. Clinical judgments require you to anticipate information, analyze the data, and make decisions regarding your patient’s care. During assessment consider all elements that build toward making an appropriate nursing diagnosis (Fig. 40-6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree