Treatment of Dehydration
Dehydration in children can be assessed by the use of tools and scales (Bailey et al. 2010). Unless treated, moderate to severe dehydration will lead to a compromise of cardiac output and systemic perfusion. If this occurs, the body uses compensatory mechanisms to try to maintain systemic and essential organ perfusion, which in turn may lead to metabolic acidosis, multi-system failure and ultimately death. These systems are interdependent (see Chapters 5 and 6).
Whether the dehydration is isotonic, hypotonic or hypertonic in origin the aim of treatment is restoration and maintenance of intravascular volume and systemic perfusion, together with correction of any electrolyte balance. In mild dehydration and, depending on cause and age of child, rehydration may be achieved through oral therapy. However, very sick children will need the insertion of one, and preferably two, wide-bore intravenous cannula. If IV access is difficult to obtain, an intraosseous needle should be considered while preparation is made for a cutdown into a vein. When access to the circulation is established, the insertions of a central venous pressure monitor should be considered.
The dehydrated child must be assessed for signs of shock. This is ascertained by clinical and biochemical examination. The cause of the dehydration also needs to be established and an effective treatment regime to allow for rehydration and correction of abnormalities in electrolyte balance over a 24–48-hour period (APLS 2005). The acid base balance also needs to be considered and treated accordingly.
The fluid resuscitation of a child in hypovolaemic shock secondary to fluid loss is the rapid administration of crystalloid. The starting volume is 20 ml/kg. This can be repeated if there is inadequate clinical response with no evidence of intravascular overload. The type of fluid used should approximate in electrolyte concentration to that of serum. Commonly used fluids are 0.9% saline or Hartmann’s solution. The presence of hyper- or hyponatraemia does not affect the choice of fluids during this stage of resuscitation (APLS 2005).
Over-Hydration and Fluid Overload
The child requiring intensive care is susceptible to fluid overload and oedema. Fluid overload is a problem as it results in more circulatory volume than the heart can effectively cope with. This may eventually result in heart failure, which typically manifests as pulmonary oedema and peripheral oedema. There should be hourly calculation of fluid input and output, continuous monitoring supplemented by continual clinical reassessment.
There are four main factors which can cause oedema (Table 12.5).
Table 12.5 Factors contributing to oedema
Source: adapted from Porth and Matfin 2004.
| Increased capillary pressure | A result of increased vascular volume caused by heart failure, kidney disease, thermal injury. A result of venous obstruction caused by acute pulmonary oedema, liver disease with portal vein congestion. A result of decreased arteriolar resistance caused by drugs blocking the calcium channels. |
| Decreased colloidal osmotic pressure | A loss of plasma proteins caused by kidney disease such as nephrotic syndrome, extensive burns. A decrease in the production of plasma proteins results in liver disease, starvation and malnutrition. |
| Increased capillary permeability | Result of extensive tissue injury or thermal injury, allergic reactions or inflammation. |
| Obstruction of lymphatic flow | Result of malignant obstruction or obliteration of lymphatic structures. |
Signs and symptoms of fluid overload include:
- Oedema.
- Tachycardia.
- Hypertension.
- Respiratory distress.
- Decrease in saturations.
- Enlarged liver.
- Raised central venous pressure.
- Weight gain.
- Signs of alveolar flooding or fluid streaks on chest X-ray.
- Frothy pink secretions if intubated.
Treatment of fluid overload will be determined by the cause, for example, if the primary cause of fluid overload is due to cardiac failure, then the use of inotropic and/or diuretic therapy may be indicated in order to increase cardiac output. However, if the fluid overload is due to over-hydration and intravascular overload, then the patient is likely to need fluid restriction and the administration of diuretics.
Electrolytes
Electrolytes are ions with the ability to conduct a charge and they account for approximately 95% of the solute molecules in body water, the other 5% being non-electrolytes such as glucose, urea and creatinine (McCance and Huether 2002). The main electrolytes are sodium, potassium, calcium, magnesium, chloride, bicarbonate and phosphate. The main intracellular electrolyte is potassium, while the main extracellular electrolyte is sodium. Normal values (Table 12.6) and daily requirements differ according to age and laboratories have slightly different reference ranges. Refer to the local policy and seek advice if unsure about a value for a particular child.
Table 12.6 Normal values of electrolytes
Source: adapted from Pearson 2002, in Dixon et al. 2009.

Electrolyte Action
Having an excess or deficiency of an electrolyte can potentially be very dangerous for any child and even more so for the child who needs intensive care.
Sodium (Na+)
As the main electrolyte in the ECF compartment Na+ has a major effect on osmosis and osmolality. Hyponatraemia is classified as a Na level <135 mmol/l. Deficits of Na alter the ability of cells to depolarise and repolarise normally.
Signs and symptoms of hyponatraemia include lethargy, headaches, seizures and coma. If hyponatraemia is accompanied by a loss of ECF, then signs of hypovolaemia may be displayed, including tachycardia, hypotension and diminished urine output. In other situations hyponatraemia may be due to water retention and detected by weight gain and oedema (McCance and Huether 2002). Treatment will depend on the cause, biochemical results and clinical manifestation. Rapid falls in sodium can be corrected quickly but these situations are unusual. Sodium levels usually fall gradually and correction should also be so. Natural corrected with fluid restriction is possible.
Hypernatraemia is a sodium level >145 mmol/l. It can be caused by excessive water loss, decreased water intake or excessive sodium intake. Signs and symptoms may include thirst, oliguria/anuria, high specific gravity of urine and increased serum osmolality. If decreased intravascular volume occurs, then tachycardia, hypotension and peripheral shutdown may also present. Neurological symptoms resulting from the movement of water out of brain cells may include headache, seizures and coma. Identification of the cause by history, clinical examination and biochemical investigations will define treatment. The cause needs to be treated but will also include rehydration.
Potassium (K+)
Potassium is the most common intracellular ion, with an intracellular concentration of 140–150 mmol/l. The extracellular levels are much lower. K+ is regulated by two main mechanisms: through the kidney, which either conserves or eliminates K+, and through the transcellular shifts between the intracellular and extracellular compartments, which allows K+ to enter body cells when plasma levels are high and move out when the plasma levels are low (Porth and Matfin 2004). The control of K+ within normal range is essential as only a slight derangement from normal levels can result in life-threatening cardiac dysrhythmias.
K+ is essential for many body functions, including growth, conduction of nerve impulses, acid base balance and the use of carbohydrates for energy. Hypokalaemia is a plasma potassium level <3.5 mmol/l. Causes of hypokalaemia include inadequate intake, excessive loss usually through gastro-intestine, skin and kidneys and redistribution of K+ between the ICF and ECF compartments.
Signs and symptoms include polyuria, vomiting, muscle cramps, confusion, metabolic alkalosis and cardiac arrhythmias. Treatment consists of treating the cause and, if necessary, administering potassium supplements either by orally or if necessary intravenously.
K+ is a powerful electrolyte. Children with abnormal K+ levels ought to be electrocardiographically monitored. Hypokalaemia can cause ECG changes, including prolonged PR interval, depression of ST segment and the flattening of the T-wave. IV K+ must be administered by a nurse or doctor who is appropriately trained and skilled in its administration. It needs to be given slowly at an infusion rate >0.4 mmol/kg/hr. K+ should only be administered through a good vascular access. The child should be ECG-monitored.
Hyperkalaemia is an increase in plasma potassium level >5 mmol/l. It is that is very rarely seen in the well child but can occur in the highly dependent patient. The main causes are excessive rapid administration of potassium, decreased renal excretion as in acute renal failure and from the movement of potassium between the ICF and ECF compartments. The signs and symptoms include nausea and diarrhoea, dizziness and muscle cramps. The main risk of hyperkalaemia is cardiac dysrhythmia, which can lead to cardiac arrest. The child should be ECG-monitored. Treatment options include decreasing or stopping intake, increasing renal excretion and increasing cellular uptake (Porth and Matfin 2004).
Calcium (Ca+), Phosphate (PO4−) and Magnesium (Mg)
Ca and Mg are closely linked to PO4 levels (Pearson 2002). They are obtained from the diet and absorbed from the intestines, with any excess eliminated in the urine. The majority of the body’s calcium and phosphate is found in bones and approximately 50 percent of magnesium is found within bones. The majority of the remaining amounts of each electrolyte is found intracellularly. Only a very small amount of calcium, magnesium and phosphate is present within the extracellular fluid and are regulated by vitamin D, parathyroid hormone and calcitonin production.
Calcium
The main source of calcium is milk and milk products. The calcium content of the bone provides strength and stability to the skeletal system. It is also used to maintain extracellular levels. The small amount of extracellular calcium is either a protein-bound complex or is ionised. It is only the ionised form that is free to leave the vascular compartment and participate in cellular function. The function of ionised calcium includes participation in enzyme reaction, an effect on membrane potential, neuronal excitability, contraction in skeletal, cardiac and smooth muscle, influences cardiac contractility and automaticity and is essential for blood clotting (Porth and Matfin 2004).
Children who are critically ill or highly dependent need are at risk of hypocalcaemia as they may have an impaired ability to gain calcium from bone stores, may have high losses from the kidney or more may bind with protein and then be unavailable. Hypocalcaemia may be acute or chronic. In the acute stage it can cause jitteriness, convulsions, ECG abnormalities, arrhythmias and heart failure. Treatment will be to identify and treat cause and by administration of calcium infusion according to local treatment regime and policy. Hypercalcaemia is rare but may occur because of excessive bone resorption as a result of cancer with bone metastases, leukaemia, lymphoma and multiple myeloma. It may occur as a result of prolonged and extensive immobilisation and in paraplegics and quadriplegics. Other causes include endocrine diseases, recovery phase of acute renal failure and Williams’ syndrome. Signs and symptoms include seizures, muscle flaccidity and tachyarrhythmias. Many patients with hypercalcaemia will be volume-depleted and will respond to fluid resuscitation followed by diuretic therapy. Other forms of treatment include renal replacement therapy (Pearson 2002).
Hypocalcaemia
Measurement of calcium can be done as total calcium or ionised calcium. The value range differs. It is important to know which one has been measured and requested.
- Total: 2–2.7 mmol/l.
- Ionised: 1.2–1.3 mmol/l.
Clinical manifestations of hypocalcaemia are due to disturbances in cellular membrane potential, resulting in neuromuscular irritability. Muscle cramps involving the back and legs are common. Insidious hypocalcaemia may produce symptoms of encephalopathy. Papilloedema occasionally occurs, and cataracts may develop after prolonged hypocalcaemia. Severe hypocalcaemia with plasma Ca <1.75 mmol/l may cause tetany, laryngospasm and generalised seizures.
Phosphate (HPO4) – Negative Electrolyte
Phosphate is essential to many bodily functions. It helps bone formation, is essential for certain metabolic processes, including the formation of ATP and the enzymes necessary for metabolism of glucose, fat and protein, it serves as an acid base buffer in ECF and in the renal excretion of hydrogen ions (Porth and Matfin 2004). Like calcium, only a small amount of phosphate is located in the ECF compartment. Causes of hypophosphataemia include decreased intestinal absorption, for example, by severe and prolonged diarrhoea, lack of vitamin D, increased renal losses and malnutrition. The treatment is by replacement therapy and treatment of the cause. The most common causes are impaired renal function and tumour lysis syndrome. If hyperphosphataemia is present, symptoms similar to hypocalcaemia will be seen.
Hyperphosphataemia
Skeletal fractures or disease, kidney failure, hypoparathyroidism, haemodialysis, diabetic ketoacidosis, acromegaly, systemic infection and intestinal obstruction can all cause phosphate retention and build-up in the blood. The disorder occurs concurrently with hypocalcaemia. Individuals with mild hyperphosphataemia are typically asymptomatic, but signs of severe hyperphosphataemia include paraesthesia, tingling in hands and fingers, muscle spasms and cramps, convulsions and cardiac arrest.
Hypophosphataemia
Low serum phosphate levels may be caused by hypomagnesaemia and hypokalaemia. Severe burns, diabetic ketoacidosis, kidney disease, hyperparathyroidism, hypothyroidism, Cushing’s syndrome, malnutrition, haemodialysis, vitamin D deficiency and prolonged diuretic therapy can also diminish blood phosphate levels. There are typically few physical signs of mild phosphate depletion. Symptoms of severe hypophosphataemia include muscle weakness, weight loss and bone deformities (osteomalacia).
Magnesium
This is a very important electrolyte. Its function includes the following: it is essential to all reactions that require ATP; and essential for replication and transcription of DNA and for the translocation of messenger RNA. It is required for cellular energy metabolism, functioning of the sodium–potassium membrane pump, nerve conduction and calcium channel activity (Porth and Matfin 2004). Magnesium is found in green vegetables, meat, nuts and seafood and body levels are mainly regulated by the kidney.
Hypomagnesaemia can be caused by impaired intake or absorption as in starvation and malabsorption. It can also be caused by increased losses as in diuretic therapy and diabetic ketoacidosis. Signs and symptoms include tetany, cardiac arrhythmias and nystagmus. Treatment is by magnesium replacement but caution is required if the intravenous route is used.
Hypermagnesaemia is rare and is mainly caused by renal failure. Signs and symptoms include lethargy, confusion, hypotension and cardiac arrhythmias. Treatment should include stopping any administration and the administration of calcium, which is an antagonist of magnesium.
Nutritional Needs of the Child
Children in the intensive care environment are uniquely vulnerable. Their nutritional care plan must match the total metabolic needs of the body and allow for repair and growth (Table 12.7). Energy input is obtained from three main classifications of food groups: carbohydrates, fats and proteins. Energy is derived from the oxidation of carbon and hydrogen from dietary molecules.
Table 12.7 Terms that are associated with metabolism include the following
| Homeostasis | The maintenance of a stable environment. |
| Anabolism | The building of large molecules from smaller ones, requiring energy, for example, making fat from fatty acids and glycerol. |
| Catabolism | The breakdown of large complex molecules into smaller ones releasing energy, for example, releasing amino acids from a peptide chain. |
| Adenosine tri-phosphate (ATP) | Energy generated and used by the cells. |
| Glycolysis | A stage in glucose metabolism where glucose is broken down resulting in the production of ATP. |
Carbohydrate Metabolism
Carbohydrates are absorbed from the digestive tract. During digestion large carbohydrate molecules, such as starch and lactose, are broken down to simple sugars, such as glucose, fructose and galactose. These are then absorbed into the bloodstream and transported to the liver by the hepatic portal vein. From the liver they are then transported systemically. Any excesses are stored as glycogen in the liver and skeletal muscle. Glycogen can be readily broken down into glucose when required. The importance of this mechanism is that some cells (e.g. brain cells) rely almost exclusively on glucose as their source of energy for ATP production.
Fat Metabolism
Fat molecules are absorbed from the digestive system in small clusters known as chylomicrons. Chylomicrons tend not to enter the blood stream directly but are absorbed by the lymphatic system. This means they are transported directly to the heart and the rest of the body. Fat consists of glycerol and fatty acids and both can be used to produce energy. Glycerol is a carbohydrate and the liver uses it to synthesise glucose. Fatty acids are fed into the Krebs cycle in the mitochondria leading to further production of ATP. Fat contains more energy per gram than carbohydrate.
Protein Metabolism
Proteins consist of chains of amino acids. These chains are broken down by enzymes in the digestive system releasing amino acids, which are then absorbed into the blood stream. Amino acids are first transported to the liver and then to the rest of the body. Amino acids are not usually used as a source of fuel, their primary function being for growth and development. However, if other sources of energy are not available, they are utilised as a source of energy. The breakdown of amino acids results in the production of ammonia, which is then converted to urea by the liver before being excreted by the kidney.
In order for the cells to be able to utilise the products of metabolism two hormones, insulin and glucagon are needed; these are secreted by the pancreas.
Insulin
Insulin is secreted by the β cells in the pancreas from the islets of Langerhans. It is a very large polypeptide and its main role is the maintenance of blood glucose by binding with large protein receptors in the cell wall. Table 12.8 reviews the action of insulin on the product of carbohydrate, fat and protein metabolism.
Table 12.8 Effects of insulin on carbohydrate, fat and protein metabolism
| Carbohydrate metabolism | Insulin is important for the transport of glucose into the adipose cells, providing the glycerol portion of the fat molecule for the depositing fat in these cells. |
| Fat metabolism | Insulin decreases the utilisation of fat as a source of energy. It inhibits the action of lipase. In the absence of insulin increased breakdown and utilisation of fat for energy occurs, which leads to excessive amounts of acetoacetic acid being formed which cannot be metabolised by the body and leads to a systemic build-up of acid. |
| Protein metabolism | Little is understood about the effects of insulin on protein synthesis and storage other than that insulin causes active transport of amino acids into the cells. Insulin has a direct effect on ribosomes by increasing the translation messenger RNA, so forming new proteins, and insulin inhibits the catabolism of proteins decreasing the rate of amino acid release from cells. |
Glucagon
Glucagon is secreted by the α cells of the islets of Langerhans in the pancreas. It has the opposite action to insulin. The effects of glucagon include the increased breakdown of glycogen stores to form glucose, the increased breakdown of fats and glucose synthesis.
Growth of the Infant and Child
Growth only occurs when more nutrition is provided than is required to meet basic metabolic needs and have a balanced intake of both macro- and micronutrients. Macronutrients include carbohydrates, lipids and proteins. Micronutrients include minerals such as calcium, magnesium, phosphate, iron, zinc and copper, water-soluble vitamins B and C and fat-soluble vitamins A, D, E and K.
It is expected that normal growth pattern would see an infant doubling its birth weight by age 6 months, trebling birth weight by 12 months and quadrupling birth weight by 24 months. Sixty per cent of children’s energy needs are utilised by their major organs in order to maintain normal function. Basal metabolic rate reaches its maximum around the age of 2 years, then decreases with age.
Nutritional Assessment
The assessment of the child’s nutritional state when first admitted to an ICU might not be one of the highest priorities, but this can contribute to the prevalence of malnutrition among critically ill patients, especially those with a protracted clinical course (Mehta and Compher 2009). Both over- and underfeeding are common. In part this is owing to the difficulties in calculating requirement as metabolic response to stress, injury, surgery or inflammation cannot be accurately predicted and the metabolic alterations may change during the course of illness (Mehta et al. 2009). Weighing patients may be difficult if they are attached to machinery and the weight recorded may not be true indication of nutritional status if they are oedematous. Calorimetry is possible but not common; illness may alter or mask biochemical results. There is a range of equations designed to calculate requirement, but none is deemed the gold standard, leading to a need for clinical creativity. Much more research is required in this area (Frankenfield and Ashcraft 2011). Enteral feeding in the highly dependent child needs to be commenced as soon as possible, interrupted and disturbed as little as possible and reviewed frequently.
Types and Methods of Feeding
Children require more calories per kg body weight than adults due to their higher metabolic rate and any child who has an illness or disease will need additional calorific intake (Singh 1997). Consideration of nutrition, including type, volume and administration of feed, needs to be made as early as possible to prevent harmful physiological effects (Alexander 1999). Enteral feeding is the method best suited to achieving maximal nutritional status in the child with high dependency needs. There are obvious advantages: it is efficient and economical, readily available and the majority of nurses have the skill and expertise needed to administer enteral feeds safely enterally. Enteral feeding maintains the gut barrier structure and function. It is an important stimulus to mature the gut of a premature infant, maximises the immunological properties of the gut and minimises the risk of bacterial translocation. However, for a number of reasons it may not be possible for the child to receive their nutritional requirement in this way. These include recent gut surgery, short gut syndrome, paralytic ileus, non-absorption of feeds, severe breathlessness and respiratory distress.
Another consideration of method and frequency of feeding is the age of the child. The volume capacity of the stomach alters with age (Table 12.9) and this, together with the condition of the child, needs to be taken into account.
Table 12.9 Stomach capacity by age
Source: Moules and Ramsey 1998.
| Age | Capacity |
| Newborn | 10–20 ml |
| 1 week | 30–90 ml |
| 2–3 weeks | 75–100 ml |
| 1 month | 90–150 ml |
| 3 months | 150–200 ml |
| 1 year | 210–360 ml |
| 2 years | 500 ml |
| 10 years | 750–900 ml |
| 16 Years | 1500 ml |
| Adulthood | 2000–3000 ml |
Whenever possible intermittent oral feeding is the preferred method, and for the infant breast milk is at least the basis of the preferred feed. However, there are many situations where oral feeding may not be an option or indeed intermittent feeding may not be appropriate, for example, where there is endotracheal intubation, absent or impaired swallowing reflexes, coma or severe breathlessness. In these situations enteral feeding can be administered by the use of naso- or orogastric tubes, nasoduodenal or nasojejunal tubes or in the situation where long-term tube feeding is anticipated, a gastrostomy or jejunostomy may be performed. Frequency of feeding can be adapted from intermittent to continuous in order to meet the individual needs and condition of the child.
Bolus or Continuous Enteral Feeding, Gastric or Transpyloric Feeding
There is controversy over commencing infants and young children on bolus or continuous feeding. Bolus is physiologically more appropriate and in the neonatal unit currently the administration of a slow bolus seems to be preferred. This style of management reflects what is done in most neonatal units, although the clinical benefits and risks of continuous versus intermittent nasogastric tube milk feeding cannot be reliably discerned from the limited information available from randomised trials (Premji and Chessell 2007). Arguments for intermittent bolus gavage include the fact that this is physiologically normal and promotes the cyclical surge of gut hormones which are normally seen in healthy term infants and gastrointestinal hormones such as gastrin, gastric inhibitory peptide and enteroglucagon require the presence of intraluminal nutrients to stimulate secretion. Continuous tube feeding has been cited as being better tolerated in some ITU patients (Rhoney et al. 2002). Continuous transpyloric (TP) feeding has been suggested as a means to manage feed intolerance (Sánchez et al. 2007) and TP enteral nutrition is a useful and simple feeding method that enables a high calorie feed to be delivered to children who are receiving high-dose sedatives and muscle relaxants (Sánchez et al. 2007). Continuous TP feeding has been cited as causing prolonged diarrhoea but is effective in maintaining nutrition and has the advantage of not contributing to gastric pooling. TP feeding permits feeding to be stopped for shorter periods prior to attempts at extubation (Babett 2007).
In the absence of evidence, from a patient safety perspective a nurse administering a bolus has to be physically present and attentive during its administration, whereas continuous feeds continue to be administered regardless of the presence of the nurse. In lightly sedated children weaning off ventilation the irritation of an NGT taped to their face may result in restlessness and partial removal. Where bolus feeding is practised this would be quickly noticed and the NGT re-passed; where continuous feeds are used it could be some time before displacement becomes apparent. The mechanism to assess the location of the tube and strategies for enteral tube management (NPSA 2011) is well documented and universally adopted and will not be considered here.
Types of Enteral Feeds
Breast milk is universally advocated as the feed of choice for infants. Current recommendations are for exclusive breast milk feeds for the first 6 months and as a dietary supplement for up to 2 years (WHO 2002). Not surprisingly it is well tolerated even in seriously compromised guts. Approximately 50% of energy in breast milk is provided by fats, whereas in infant formulas it is approximately 25%. Breast milk has protective properties (e.g. immunoglobulin A − IgA) and has antibodies which are active against a wide range of bacterial, viral, fungal and parasitic antigens. Lactoferrin binds with free iron in milk and is thought to inhibit the growth of E. coli and lysosome which attacks bacteria membranes.
Growth factors in breast milk may have a role in maintaining the protective nature of the intact gastrointestinal mucosa as breast milk promotes the growth of normal gut flora. Breast-fed infants are less likely to experience infections compared to formula-fed infants, particularly where there are risks of unhygienic preparation and storage of the feed. For the infants of mothers who were unable to express breast milk or who are unable to express sufficient milk to feed their infant, donor milk is becoming more readily available.
Most infant feeding formulas are based on cow’s milk which has been modified to resemble the nutritional composition of mature breast milk. Modified cow’s milk feeds can be divided into three groups (Table 12.10):
- Whey-dominant – whey:casein ratio similar to breast milk, e.g. Aptamil First™, Cow & Gate Premium™, Farley’s First Milk™, SMA Gold™.
- Casein-dominant – whey:casein ratio similar to cow’s milk, there is no scientific evidence to support the claim that a casein-dominant feed will help to satisfy a hungry infant.
- Follow-on formula for infants over 6 months of age has a higher iron content.
Table 12.10 Types of formula feed
| Age | Type of formula feed |
| Preterm <2 kg | Nutriprem SMA low birth weight |
| Preterm >2 kg | Nutriprem 2, Premcare |
| Full term and under 1year | EBM, SMA Gold, C&G Premium or Plus, Aptimil, Farley’s First |
| 1–6 years (8–20 kg) | Nutrini, Nutrini-fibre, Nutrini-energy Multifibre |
| 7–12 (21–45 kg) | Tentrini Multifibre, Tentrini energy with fibre |
| 13 years+ | Nutrison Multifibre |
The Best Enteral Feed for the Child
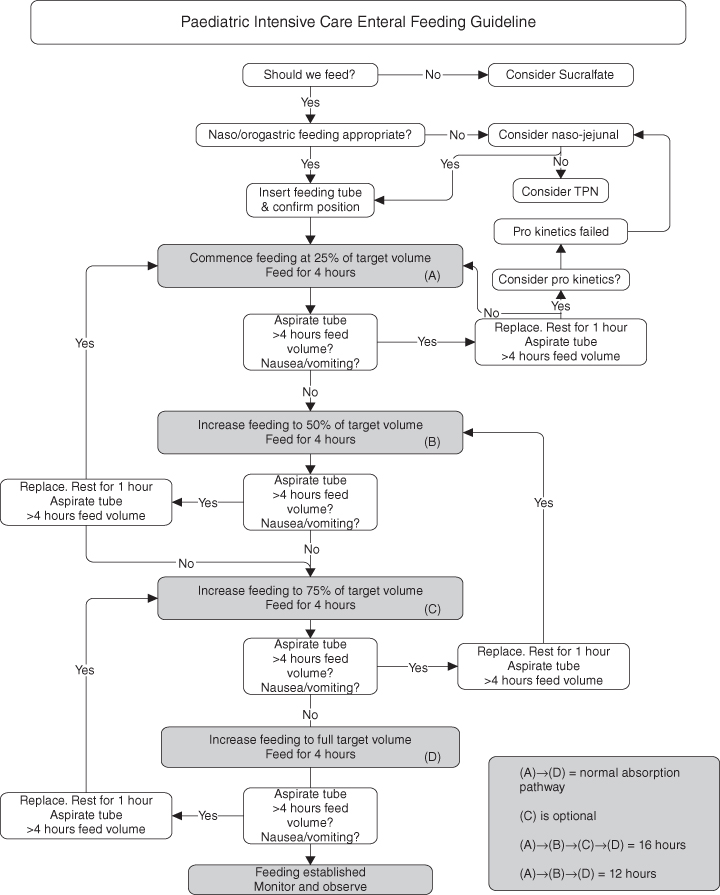
Consideration of the type of enteral feed used should include patient condition, availability of feed and parental choice. Dieticians should be involved in ensuring that high dependency patients are receiving the appropriate feed, including any additional supplements, in order to meet the growth and metabolic needs of the child. Types of enteral feed available are listed in Tables 12.11 and a strategy by which the enteral feed can be commenced and built up is considered in Figure 12.1.
Table 12.11 Types of enteral feed
| Oral supplements | Not complete diets but used to supply more energy. |
| Polymeric diets | For children with normal/near-normal gut function. Contains protein, long-chain fatty acids, complex carbohydrates, vitamins and minerals (e.g. Pediasure and Ensure). |
| Pre-digested diets | For children with severely impaired gastrointestinal function. Contains free amino acids, di- and tripeptides, short carbohydrate polymers and fat, as well as vitamins, minerals and essential fatty acids (e.g. Pregestimil). |
| Elemental diets | For patients with severe gastrointestinal dysfunction. |
| Disease-specific diets | Diets formulated for specific conditions such as renal failure, hepatic encephalopathy, chylothorax. |
| Modular diets | Individual components of a feed can be adjusted to achieve optimum diet. |
Figure 12.1 Progressing feeds.
From Martin and Cox 2000.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


