Chapter 67 1. Relate the pathophysiology to the clinical manifestations of the different types of shock: cardiogenic, hypovolemic, distributive, and obstructive. 2. Compare the effects of shock, systemic inflammatory response syndrome, and multiple organ dysfunction syndrome on the major body systems. 3. Compare the collaborative care, drug therapy, and nursing management of patients experiencing different types of shock. 4. Describe the nursing management of a patient experiencing multiple organ dysfunction syndrome. Shock, systemic inflammatory response syndrome (SIRS), and multiple organ dysfunction syndrome (MODS) are serious and interrelated problems (Fig. 67-1). This chapter provides an overview of the different types of shock, SIRS, and MODS, and the related management of each. The four main categories of shock are cardiogenic, hypovolemic, distributive, and obstructive1,2 (Table 67-1). Although the cause, initial presentation, and management strategies vary for each type of shock, the physiologic responses of the cells to hypoperfusion are similar. TABLE 67-1 CLASSIFICATION OF SHOCK STATES Cardiogenic shock occurs when either systolic or diastolic dysfunction of the heart’s pumping action results in reduced cardiac output (CO). Causes of cardiogenic shock are listed in Table 67-1. Mortality rates for patients with cardiogenic shock approach 60%.3 Decreased filling of the heart results in decreased stroke volume. The heart’s inability to pump the blood forward is called systolic dysfunction. Systolic dysfunction primarily affects the left ventricle, since systolic pressure is greater on the left side of the heart. When systolic dysfunction affects the right side of the heart, blood flow through the pulmonary circulation is reduced. The most common cause of systolic dysfunction is acute myocardial infarction (MI). Cardiogenic shock is the leading cause of death from acute MI.4 Causes of diastolic dysfunction are listed in Table 67-1. Fig. 67-2 describes the pathophysiology of cardiogenic shock. Whether the initiating event is myocardial ischemia, a structural problem (e.g., valvular disorder, ventricular septal rupture), or dysrhythmias, the physiologic responses are similar: the patient experiences impaired tissue perfusion and cellular metabolism. The early clinical presentation of a patient with cardiogenic shock is similar to that of a patient with acute decompensated heart failure (see Chapter 35). The patient may have tachycardia and hypotension. Pulse pressure may be narrowed due to the heart’s inability to pump blood forward during systole and increased volume during diastole. An increase in systemic vascular resistance (SVR) increases the workload of the heart, thus increasing the myocardial oxygen consumption. The heart’s inability to pump blood forward also results in a low CO (less than 4 L/minute) and cardiac index (less than 2.5 L/min/m2). Signs of peripheral hypoperfusion (e.g., cyanosis, pallor, diaphoresis, weak peripheral pulses, cool and clammy skin, delayed capillary refill) are seen. Decreased renal blood flow results in sodium and water retention and decreased urine output. Anxiety, confusion, and agitation may develop as cerebral perfusion is impaired. Tables 67-2 and 67-3 describe the laboratory findings and clinical presentation of a patient with cardiogenic shock. TABLE 67-2 DIAGNOSTIC STUDIES TABLE 67-3 CLINICAL PRESENTATION OF TYPES OF SHOCK ARDS, Acute respiratory distress syndrome; BUN, blood urea nitrogen; LOC, level of consciousness; Hypovolemic shock occurs after a loss of intravascular fluid volume (see Table 67-1). The volume is inadequate to fill the vascular space. The volume loss may be either an absolute or a relative volume loss. Absolute hypovolemia results when fluid is lost through hemorrhage, gastrointestinal (GI) loss (e.g., vomiting, diarrhea), fistula drainage, diabetes insipidus, or diuresis. In relative hypovolemia, fluid volume moves out of the vascular space into the extravascular space (e.g., intracavitary space). This type of fluid shift is called third spacing. One example of relative volume loss is leakage of fluid from the vascular space to the interstitial space from increased capillary permeability, as seen in burns (see Chapter 25). In hypovolemic shock the size of the vascular compartment remains unchanged while the volume of blood or plasma decreases. Whether the loss of intravascular volume is absolute or relative, the physiologic consequences are similar. A reduction in intravascular volume results in a decreased venous return to the heart, decreased preload, decreased stroke volume, and decreased CO. A cascade of events results in decreased tissue perfusion and impaired cellular metabolism, the hallmarks of shock (Fig. 67-3). The patient’s response to acute volume loss depends on a number of factors, including extent of injury, age, and general state of health. However, the clinical presentation of hypovolemic shock is consistent (see Table 67-3). An overall assessment of physiologic reserves may indicate the patient’s ability to compensate. A patient may compensate for a loss of up to 15% of the total blood volume (approximately 750 mL). Further loss of volume (15% to 30%) results in a sympathetic nervous system (SNS)–mediated response. This response results in an increase in heart rate, CO, and respiratory rate and depth. The stroke volume, central venous pressure (CVP), and PAWP are decreased because of the decreased circulating blood volume. The patient may appear anxious, and urine output begins to decrease. If hypovolemia is corrected by crystalloid fluid replacement at this time, tissue dysfunction is generally reversible. If volume loss is greater than 30%, compensatory mechanisms may begin to fail and immediate replacement with blood products should be started. Loss of autoregulation in the microcirculation and irreversible tissue destruction occur with loss of more than 40% of the total blood volume.5,6 Common laboratory studies and assessments that are done include serial measurements of hemoglobin and hematocrit levels, electrolytes, lactate, blood gases, mixed central venous oxygen saturation (SvO2), and hourly urine outputs (see Table 67-2). Neurogenic shock is a hemodynamic phenomenon that can occur within 30 minutes of a spinal cord injury at the fifth thoracic (T5) vertebra or above; it can last up to 6 weeks. The injury results in a massive vasodilation without compensation because of the loss of SNS vasoconstrictor tone. This massive vasodilation leads to a pooling of blood in the blood vessels, tissue hypoperfusion, and ultimately impaired cellular metabolism (Fig. 67-4). In addition to spinal cord injury, spinal anesthesia can block transmission of impulses from the SNS. Depression of the vasomotor center of the medulla from drugs (e.g., opioids, benzodiazepines) also can decrease the vasoconstrictor tone of the peripheral blood vessels, resulting in neurogenic shock (see Table 67-1). The most important clinical manifestations in neurogenic shock are hypotension (from the massive vasodilation) and bradycardia (from unopposed parasympathetic stimulation).7 The patient may not be able to regulate body temperature. Combined with massive vasodilation, the inability to regulate temperature promotes heat loss. Initially, the patient’s skin is warm due to the massive dilation. As the heat disperses, the patient is at risk for hypothermia. Later, the patient’s skin may be cool or warm depending on the ambient temperature (poikilothermia—taking on the temperature of the environment). In either case, the skin is usually dry. Tables 67-2 and 67-3 further describe the laboratory findings and clinical presentation of a patient with neurogenic shock. Although spinal shock and neurogenic shock often occur in the same patient, they are not the same disorder. Spinal shock is a transient condition that is present after an acute spinal cord injury (see Chapter 61). The patient with spinal shock experiences the absence of all voluntary and reflex neurologic activity below the level of the injury. Anaphylactic shock is an acute, life-threatening hypersensitivity (allergic) reaction to a sensitizing substance (e.g., drug, chemical, vaccine, food, insect venom). The reaction quickly causes massive vasodilation, release of vasoactive mediators, and an increase in capillary permeability. As capillary permeability increases, fluid leaks from the vascular space into the interstitial space. Anaphylactic shock can lead to respiratory distress due to laryngeal edema or severe bronchospasm, and circulatory failure from the massive vasodilation.8 The patient has a sudden onset of symptoms, including dizziness, chest pain, incontinence, swelling of the lips and tongue, wheezing, and stridor. Skin changes include flushing, pruritus, urticaria, and angioedema. In addition, the patient may be anxious and confused and have a sense of impending doom. A patient can have a severe allergic reaction, possibly leading to anaphylactic shock, after contact, inhalation, ingestion, or injection with an antigen (allergen) to which the individual has previously been sensitized (see Table 67-1). Parenteral administration of the antigen (allergen) is the route most likely to cause anaphylaxis. However, oral, topical, and inhalation routes can also cause anaphylactic reactions. Tables 67-2 and 67-3 describe the laboratory findings and clinical presentation of a patient in anaphylactic shock. Quick and decisive action is critical to prevent the progression of an allergic reaction to anaphylactic shock. (Anaphylaxis is discussed in Chapter 14.) Sepsis is a systemic inflammatory response to a documented or suspected infection (Table 67-4). In as many as 10% to 30% of patients with sepsis, the causative organism is not identified. Severe sepsis, defined as sepsis complicated by organ dysfunction, is diagnosed in more than 750,000 patients per year and has mortality rates as high as 28% to 50%.9–11 TABLE 67-4 DIAGNOSTIC CRITERIA FOR SEPSIS Source: Dellinger RP, Levy MM, Rhodes A, et al: Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012, Crit Care Med 41:580, 2013. Septic shock is the presence of sepsis with hypotension despite adequate fluid resuscitation, along with inadequate tissue perfusion resulting in tissue hypoxia. The main organisms that cause sepsis are gram-negative and gram-positive bacteria. Parasites, fungi, and viruses can also cause sepsis and septic shock.12 Fig. 67-5 presents the pathogenesis of septic shock. When a microorganism enters the body, the normal immune or inflammatory responses are started. However, in severe sepsis and septic shock the body’s response to the microorganism is exaggerated. Inflammation and coagulation increase, and fibrinolysis decreases. Endotoxins from the microorganism cell wall stimulate the release of cytokines, including tumor necrosis factor (TNF), interleukin-1 (IL-1), and other proinflammatory mediators that act through secondary mediators such as platelet-activating factor, IL-6, and IL-8.12 (See Chapter 12 for discussion of the inflammatory response.) The release of platelet-activating factor results in the formation of microthrombi and obstruction of the microvasculature. The combined effects of the mediators result in damage to the endothelium, vasodilation, increased capillary permeability, and neutrophil and platelet aggregation and adhesion to the endothelium. In addition to the cardiovascular dysfunction that accompanies sepsis, respiratory failure is common. The patient initially hyperventilates as a compensatory mechanism, resulting in respiratory alkalosis. Once the patient can no longer compensate, respiratory acidosis develops. Respiratory failure develops in 85% of patients with sepsis, and 40% develop acute respiratory distress syndrome (ARDS) (see Chapter 68). These patients need to be intubated and mechanically ventilated. Other clinical signs of septic shock include alteration in neurologic status; decreased urine output; and GI dysfunction, such as GI bleeding and paralytic ileus. Table 67-3 gives the clinical presentation of a patient with septic shock. Obstructive shock develops when a physical obstruction to blood flow occurs with a decreased CO (Fig. 67-6). This can be caused by restricted diastolic filling of the right ventricle from compression (e.g., cardiac tamponade, tension pneumothorax, superior vena cava syndrome). Other causes include abdominal compartment syndrome in which increased abdominal pressures compress the inferior vena cava, thus decreasing venous return to the heart (see Chapter 43). Pulmonary embolism and right ventricular thrombi cause an outflow obstruction as blood leaves the right ventricle through the pulmonary artery. This leads to decreased blood flow to the lungs and decreased blood return to the left atrium. Patients experience a decreased CO, increased afterload, and variable left ventricular filling pressures depending on the obstruction. Other clinical signs include jugular venous distention and pulsus paradoxus (see Table 37-5). Rapid assessment and treatment are important to prevent further hemodynamic compromise and possibly cardiac arrest (see Fig. 67-6). In addition to an understanding of the underlying pathogenesis of the type of shock that the patient is experiencing, monitoring and management are guided by knowing where the patient is on the shock “continuum.” This continuum begins with the initial stage of shock that occurs at a cellular level and is usually not clinically apparent. Metabolism changes at the cellular level from aerobic to anaerobic, causing lactic acid buildup. Lactic acid is a waste product that is removed by the liver. However, this process requires oxygen, which is unavailable because of the decrease in tissue perfusion. Shock is categorized into three clinically apparent but overlapping stages: compensatory stage, progressive stage, and irreversible stage.5 In the compensatory stage the body activates neural, hormonal, and biochemical compensatory mechanisms in an attempt to overcome the increasing consequences of anaerobic metabolism and to maintain homeostasis. (See eFig. 67-1 on the website for this chapter.) The patient’s clinical presentation begins to reflect the body’s responses to the imbalance in oxygen supply and demand (Table 67-5). TABLE 67-5 MANIFESTATIONS OF STAGES OF SHOCK*
Nursing Management
Shock, Systemic Inflammatory Response Syndrome, and Multiple Organ Dysfunction Syndrome
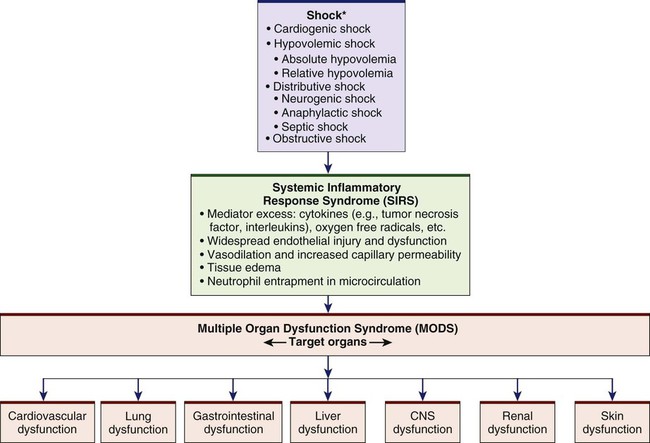
Shock
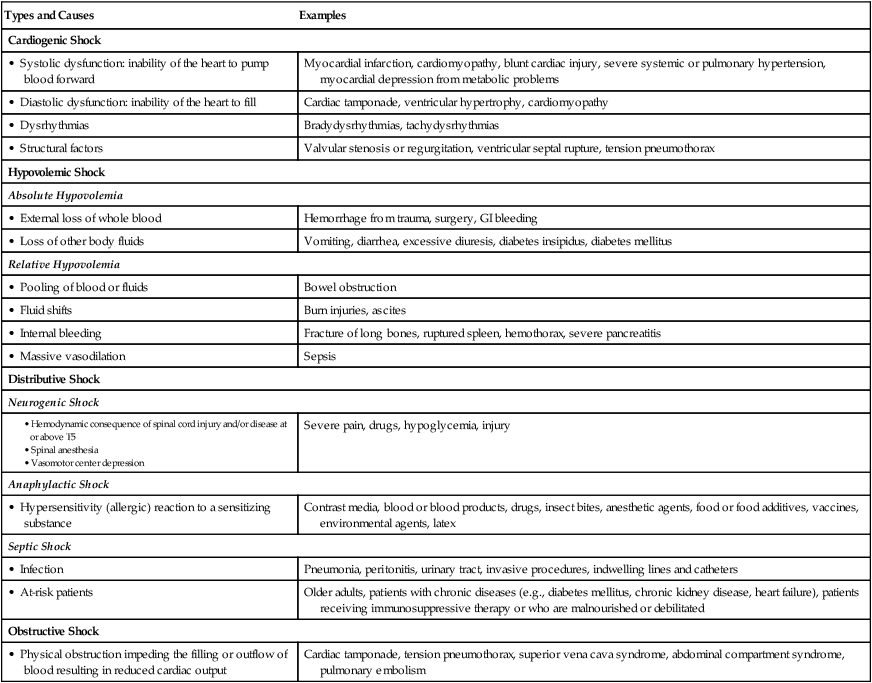
Classification of Shock
Types and Causes
Examples
Cardiogenic Shock
• Systolic dysfunction: inability of the heart to pump blood forward
Myocardial infarction, cardiomyopathy, blunt cardiac injury, severe systemic or pulmonary hypertension, myocardial depression from metabolic problems
• Diastolic dysfunction: inability of the heart to fill
Cardiac tamponade, ventricular hypertrophy, cardiomyopathy
• Dysrhythmias
Bradydysrhythmias, tachydysrhythmias
• Structural factors
Valvular stenosis or regurgitation, ventricular septal rupture, tension pneumothorax
Hypovolemic Shock
Absolute Hypovolemia
• External loss of whole blood
Hemorrhage from trauma, surgery, GI bleeding
• Loss of other body fluids
Vomiting, diarrhea, excessive diuresis, diabetes insipidus, diabetes mellitus
Relative Hypovolemia
• Pooling of blood or fluids
Bowel obstruction
• Fluid shifts
Burn injuries, ascites
• Internal bleeding
Fracture of long bones, ruptured spleen, hemothorax, severe pancreatitis
• Massive vasodilation
Sepsis
Distributive Shock
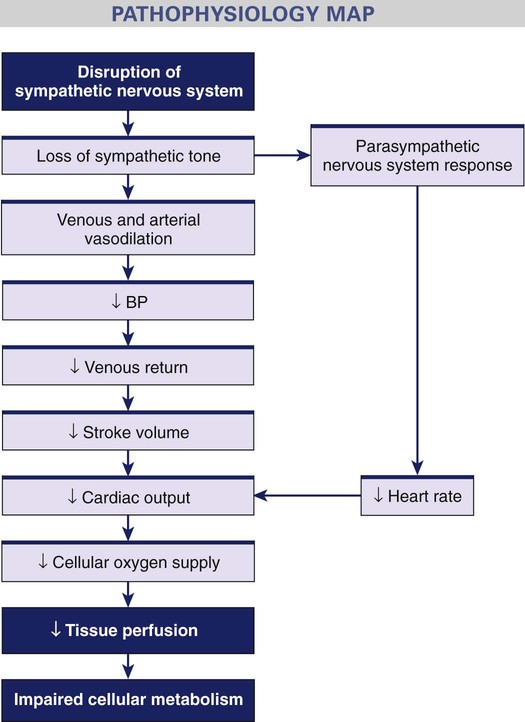
Neurogenic Shock
Severe pain, drugs, hypoglycemia, injury
Anaphylactic Shock
• Hypersensitivity (allergic) reaction to a sensitizing substance
Contrast media, blood or blood products, drugs, insect bites, anesthetic agents, food or food additives, vaccines, environmental agents, latex
Septic Shock
• Infection
Pneumonia, peritonitis, urinary tract, invasive procedures, indwelling lines and catheters
• At-risk patients
Older adults, patients with chronic diseases (e.g., diabetes mellitus, chronic kidney disease, heart failure), patients receiving immunosuppressive therapy or who are malnourished or debilitated
Obstructive Shock
• Physical obstruction impeding the filling or outflow of blood resulting in reduced cardiac output
Cardiac tamponade, tension pneumothorax, superior vena cava syndrome, abdominal compartment syndrome, pulmonary embolism
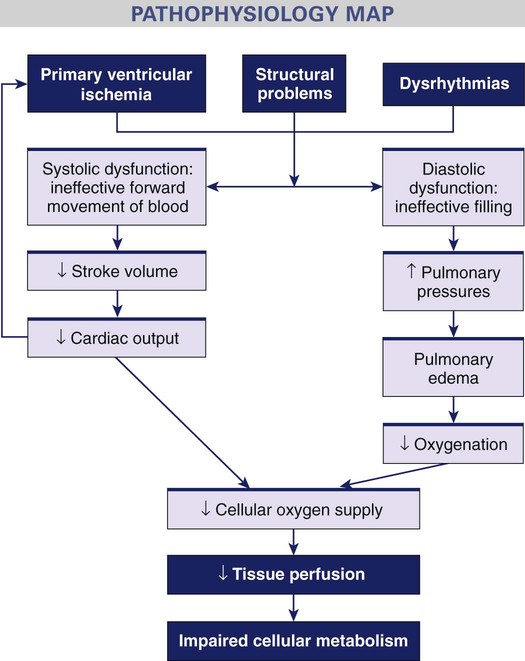
Cardiogenic Shock.
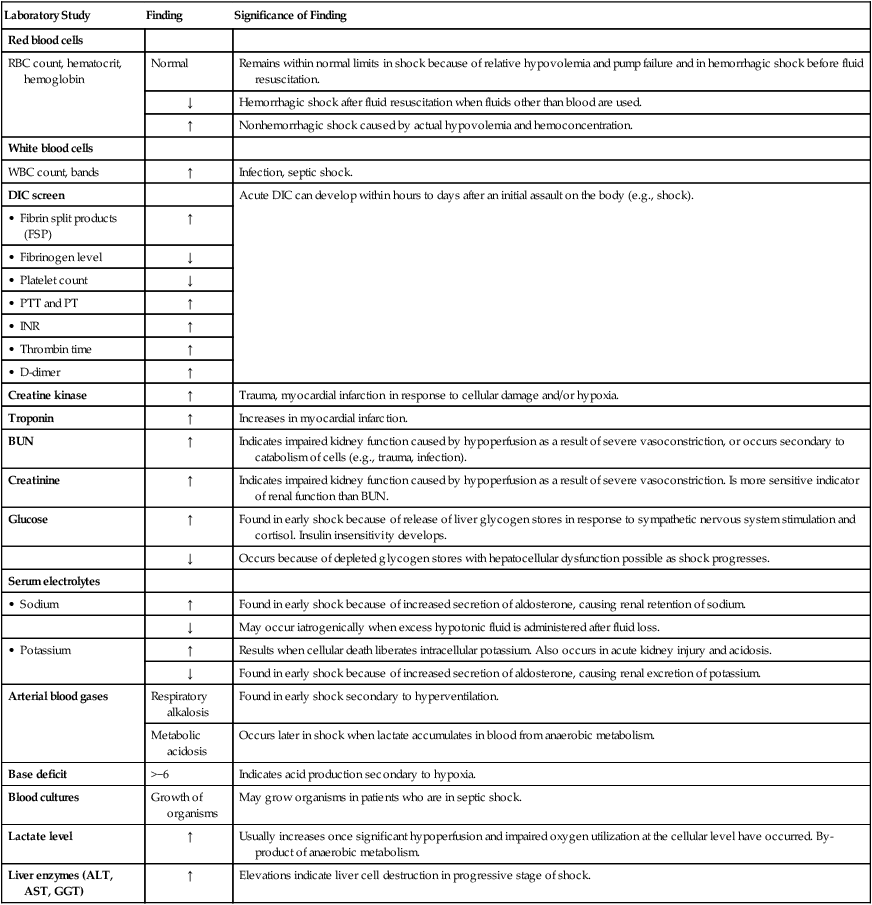
Laboratory Changes in Shock
Laboratory Study
Finding
Significance of Finding
Red blood cells
RBC count, hematocrit, hemoglobin
Normal
Remains within normal limits in shock because of relative hypovolemia and pump failure and in hemorrhagic shock before fluid resuscitation.
↓
Hemorrhagic shock after fluid resuscitation when fluids other than blood are used.
↑
Nonhemorrhagic shock caused by actual hypovolemia and hemoconcentration.
White blood cells
WBC count, bands
↑
Infection, septic shock.
DIC screen
Acute DIC can develop within hours to days after an initial assault on the body (e.g., shock).
• Fibrin split products (FSP)
↑
• Fibrinogen level
↓
• Platelet count
↓
• PTT and PT
↑
• INR
↑
• Thrombin time
↑
• D-dimer
↑
Creatine kinase
↑
Trauma, myocardial infarction in response to cellular damage and/or hypoxia.
Troponin
↑
Increases in myocardial infarction.
BUN
↑
Indicates impaired kidney function caused by hypoperfusion as a result of severe vasoconstriction, or occurs secondary to catabolism of cells (e.g., trauma, infection).
Creatinine
↑
Indicates impaired kidney function caused by hypoperfusion as a result of severe vasoconstriction. Is more sensitive indicator of renal function than BUN.
Glucose
↑
Found in early shock because of release of liver glycogen stores in response to sympathetic nervous system stimulation and cortisol. Insulin insensitivity develops.
↓
Occurs because of depleted glycogen stores with hepatocellular dysfunction possible as shock progresses.
Serum electrolytes
• Sodium
↑
Found in early shock because of increased secretion of aldosterone, causing renal retention of sodium.
↓
May occur iatrogenically when excess hypotonic fluid is administered after fluid loss.
• Potassium
↑
Results when cellular death liberates intracellular potassium. Also occurs in acute kidney injury and acidosis.
↓
Found in early shock because of increased secretion of aldosterone, causing renal excretion of potassium.
Arterial blood gases
Respiratory alkalosis
Found in early shock secondary to hyperventilation.
Metabolic acidosis
Occurs later in shock when lactate accumulates in blood from anaerobic metabolism.
Base deficit
>−6
Indicates acid production secondary to hypoxia.
Blood cultures
Growth of organisms
May grow organisms in patients who are in septic shock.
Lactate level
↑
Usually increases once significant hypoperfusion and impaired oxygen utilization at the cellular level have occurred. By-product of anaerobic metabolism.
Liver enzymes (ALT, AST, GGT)
↑
Elevations indicate liver cell destruction in progressive stage of shock.
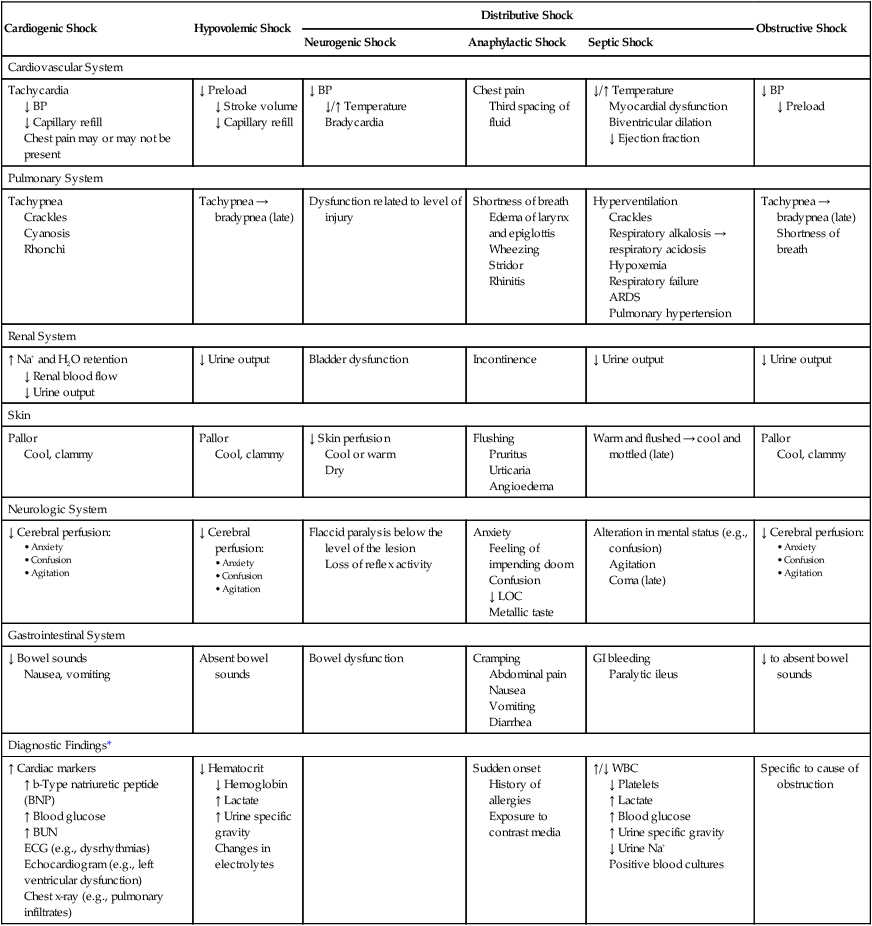
Cardiogenic Shock
Hypovolemic Shock
Distributive Shock
Obstructive Shock
Neurogenic Shock
Anaphylactic Shock
Septic Shock
Cardiovascular System
Tachycardia
↓ BP
↓ Capillary refill
Chest pain may or may not be present
↓ Preload
↓ Stroke volume
↓ Capillary refill
↓ BP
↓/↑ Temperature
Bradycardia
Chest pain
Third spacing of fluid
↓/↑ Temperature
Myocardial dysfunction
Biventricular dilation
↓ Ejection fraction
↓ BP
↓ Preload
Pulmonary System
Tachypnea
Crackles
Cyanosis
Rhonchi
Tachypnea → bradypnea (late)
Dysfunction related to level of injury
Shortness of breath
Edema of larynx and epiglottis
Wheezing
Stridor
Rhinitis
Hyperventilation
Crackles
Respiratory alkalosis → respiratory acidosis
Hypoxemia
Respiratory failure
ARDS
Pulmonary hypertension
Tachypnea → bradypnea (late)
Shortness of breath
Renal System
↑ Na+ and H2O retention
↓ Renal blood flow
↓ Urine output
↓ Urine output
Bladder dysfunction
Incontinence
↓ Urine output
↓ Urine output
Skin
Pallor
Cool, clammy
Pallor
Cool, clammy
↓ Skin perfusion
Cool or warm
Dry
Flushing
Pruritus
Urticaria
Angioedema
Warm and flushed → cool and mottled (late)
Pallor
Cool, clammy
Neurologic System
↓ Cerebral perfusion:
↓ Cerebral perfusion:
Flaccid paralysis below the level of the lesion
Loss of reflex activity
Anxiety
Feeling of impending doom
Confusion
↓ LOC
Metallic taste
Alteration in mental status (e.g., confusion)
Agitation
Coma (late)
↓ Cerebral perfusion:
Gastrointestinal System
↓ Bowel sounds
Nausea, vomiting
Absent bowel sounds
Bowel dysfunction
Cramping
Abdominal pain
Nausea
Vomiting
Diarrhea
GI bleeding
Paralytic ileus
↓ to absent bowel sounds
Diagnostic Findings*
↑ Cardiac markers
↑ b-Type natriuretic peptide (BNP)
↑ Blood glucose
↑ BUN
ECG (e.g., dysrhythmias)
Echocardiogram (e.g., left ventricular dysfunction)
Chest x-ray (e.g., pulmonary infiltrates)
↓ Hematocrit
↓ Hemoglobin
↑ Lactate
↑ Urine specific gravity
Changes in electrolytes
Sudden onset
History of allergies
Exposure to contrast media
↑/↓ WBC
↓ Platelets
↑ Lactate
↑ Blood glucose
↑ Urine specific gravity
↓ Urine Na+
Positive blood cultures
Specific to cause of obstruction
Hypovolemic Shock.
Distributive Shock
Neurogenic Shock.
Anaphylactic Shock.
Septic Shock.
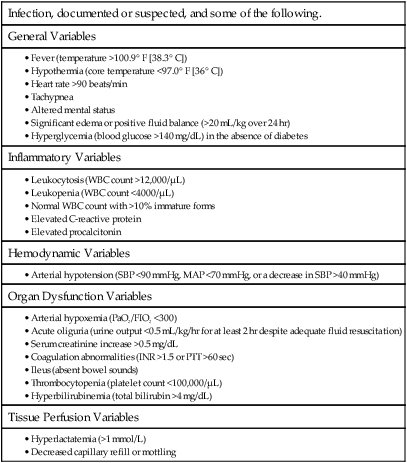
Infection, documented or suspected, and some of the following.
General Variables
Inflammatory Variables
Hemodynamic Variables
Organ Dysfunction Variables
Tissue Perfusion Variables
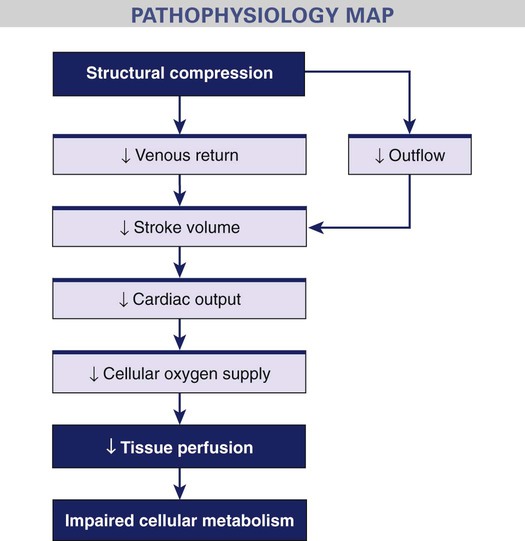
Obstructive Shock.
Stages of Shock
Compensatory Stage.
Compensatory Stage
Progressive Stage
Irreversible Stage
Neurologic System
Oriented to person, place, time
Restless, apprehensive, confused
Change in level of consciousness
↓ Cerebral perfusion pressure
↓ Cerebral blood flow
↓ Responsiveness to stimuli
Delirium
Unresponsive
Areflexia (loss of reflexes)
Pupils nonreactive and dilated
Cardiovascular System
Sympathetic nervous system response:
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

Nursing Management: Shock, Systemic Inflammatory Response Syndrome, and Multiple Organ Dysfunction Syndrome
Get Clinical Tree app for offline access