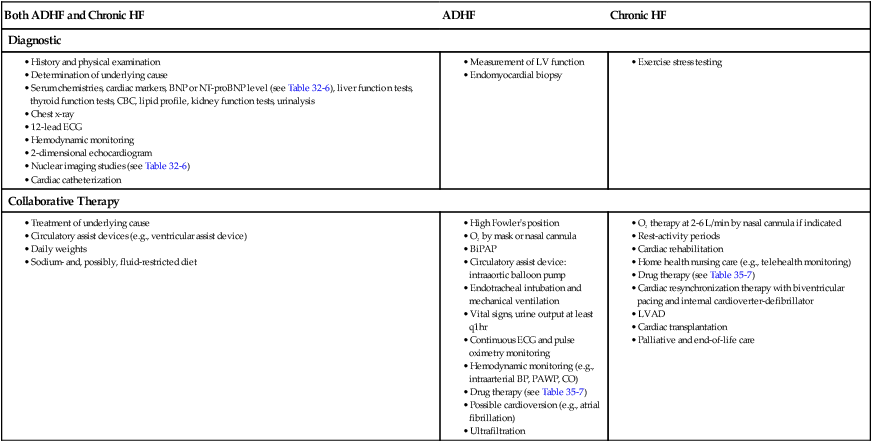
Chapter 35 1. Compare the pathophysiology of systolic and diastolic ventricular failure. 2. Relate the compensatory mechanisms involved in heart failure (HF) to the development of acute decompensated heart failure (ADHF) and chronic HF. 3. Select appropriate nursing and collaborative interventions to manage the patient with ADHF. 4. Select appropriate nursing and collaborative interventions to manage the patient with chronic HF. 5. Describe the indications for cardiac transplantation and the nursing management of cardiac transplant recipients. HF causes the heart to be unable to provide sufficient blood to meet the oxygen needs of the tissues. In clinical practice, the terms acute and chronic HF have replaced the term congestive HF (CHF) because not all HF involves pulmonary congestion. However, the term CHF is still commonly used.1,2 HF is associated with numerous cardiovascular diseases, particularly long-standing hypertension, coronary artery disease (CAD), and myocardial infarction (MI). Unlike other cardiovascular diseases, HF is increasing in incidence and prevalence. This is due to improved survival after cardiac events and the growing aging population. Currently, about 5.1 million people in the United States have HF. The American Heart Association (AHA) estimates that over 600,000 new cases are diagnosed each year. HF is primarily a disease of older adults; approximately 10 in every 1000 persons over the age of 65 have HF. The incidence of HF is similar in men and women.3 HF is the most common reason for hospital admission in older adults. This places a significant economic burden on the health care system.1 The complex, progressive nature of HF often results in poor outcomes, the most costly being hospital readmissions.3 HF may be caused by any interference with the normal mechanisms regulating cardiac output (CO). CO depends on (1) preload, (2) afterload, (3) myocardial contractility, and (4) heart rate (HR). (Preload and afterload are discussed in Chapter 32.) Any changes in these factors can lead to decreased ventricular function and HF. The major causes of HF may be divided into two subgroups: (1) primary causes (Table 35-1) and (2) precipitating causes (Table 35-2). Precipitating causes often increase the workload of the ventricles, resulting in an acute condition and decreased heart function. TABLE 35-1 PRIMARY CAUSES OF HEART FAILURE TABLE 35-2 PRECIPITATING CAUSES OF HEART FAILURE* CO, Cardiac output; LV, left ventricle; RV, right ventricle. Specific genes and gene mutations have been linked to the development of hypertension, CAD, and cardiomyopathy (weakening of the heart muscle) (see Chapters 33, 34, and 37). These cardiovascular diseases are known risk factors for HF. Knowledge of why some people with these diseases are at high risk for developing HF is incomplete. Future research into the effects of these gene mutations will most likely be related to the common causes of HF.4 Diastolic failure is the inability of the ventricles to relax and fill during diastole. Diastolic failure is often referred to as HF with normal EF. Decreased filling of the ventricles results in decreased stroke volume and CO. Diastolic failure is characterized by high filling pressures because of stiff ventricles. This results in venous engorgement in both the pulmonary and systemic vascular systems. Diastolic failure is usually the result of left ventricular hypertrophy from hypertension (most common), myocardial ischemia, valve disease (e.g., aortic, mitral), or cardiomyopathy. However, many patients do not have an identifiable heart disease. The diagnosis of diastolic failure is made based on the presence of HF symptoms with a normal EF.5 Diastolic failure occurs more frequently in older adults, women, and people who are obese (see Gender Differences box). Mixed systolic and diastolic failure is seen in disease states such as dilated cardiomyopathy (DCM). DCM is a condition in which poor systolic function is further compromised by dilated left ventricular walls that are unable to relax (see Chapter 37). These patients often have extremely low EFs (less than 35%), high pulmonary pressures, and biventricular failure (both ventricles are dilated and have poor filling and emptying capacity). As the CO falls, blood flow to the kidneys decreases. This is sensed by the juxtaglomerular apparatus in the kidneys as decreased volume. In response, the kidneys release renin, which converts angiotensinogen to angiotensin I (see Chapter 45 and Fig. 45-4). Angiotensin I is subsequently converted to angiotensin II by a converting enzyme made in the lungs. Angiotensin II causes (1) the adrenal cortex to release aldosterone, which results in sodium and water retention; and (2) increased peripheral vasoconstriction, which increases BP. This response is known as the renin-angiotensin-aldosterone system (RAAS). Dilation is an enlargement of the chambers of the heart (Fig. 35-1, A). It occurs when pressure in the heart chambers (usually the LV) is elevated over time. The heart muscle fibers stretch in response to the volume of blood in the heart at the end of diastole. The degree of stretch is directly related to the force of the contraction (systole) (this is the Frank-Starling law). This increased contraction initially leads to increased CO and maintenance of BP and perfusion. Dilation starts as an adaptive mechanism to cope with increasing blood volume. Eventually this mechanism becomes inadequate because the elastic elements of the muscle fibers are overstretched and can no longer contract effectively, thereby decreasing the CO. Hypertrophy is an increase in the muscle mass and cardiac wall thickness in response to overwork and strain (Fig. 35-1, B). It occurs slowly because it takes time for this increased muscle tissue to develop. Initially, the increased contractile power of the muscle fibers leads to an increase in CO and maintenance of tissue perfusion. Over time, hypertrophic heart muscle has poor contractility, requires more oxygen to perform work, has poor coronary artery circulation (tissue becomes ischemic more easily), and is prone to dysrhythmias. The body’s attempts to maintain balance are demonstrated by several counterregulatory processes. Natriuretic peptides (atrial natriuretic peptide [ANP] and brain, or b-type, natriuretic peptide [BNP]) are hormones produced by the heart muscle. ANP is released from the atria and BNP is released from the ventricles in response to increased blood volume in the heart.6 The natriuretic peptides have renal, cardiovascular, and hormonal effects. Renal effects include (1) increased glomerular filtration rate and diuresis and (2) excretion of sodium (natriuresis). Cardiovascular effects include vasodilation and decreased BP. Hormonal effects include (1) inhibition of aldosterone and renin secretion and (2) interference with ADH release. The combined effects of ANP and BNP help to counter the adverse effects of the SNS and RAAS in patients with HF.5 HF is usually manifested by biventricular failure, although one ventricle may precede the other in dysfunction. Normally the pumping actions of the left and right sides of the heart are synchronized, producing a continuous flow of blood. However, as a result of pathologic conditions, one side may fail while the other side continues to function normally for a time. Because of the prolonged strain, both sides of the heart eventually fail, resulting in biventricular failure (Fig. 35-2). In acute decompensated HF (ADHF), an increase in the pulmonary venous pressure is caused by failure of the LV. This results in engorgement of the pulmonary vascular system (Fig. 35-3, A and B). As a result, the lungs become less compliant, and there is increased resistance in the small airways. In addition, the lymphatic system increases its flow to help maintain a constant volume of the pulmonary extravascular fluid. This early stage is clinically associated with a mild increase in the respiratory rate and a decrease in partial pressure of oxygen in arterial blood (PaO2). If pulmonary venous pressure continues to increase, the increase in intravascular pressure causes more fluid to move into the interstitial space than the lymphatics can remove. Interstitial edema occurs at this point (Fig. 35-3, C). Tachypnea develops, and the patient becomes symptomatic (e.g., short of breath). If the pulmonary venous pressure increases further, the alveoli lining cells are disrupted and a fluid containing red blood cells (RBCs) moves into the alveoli (alveolar edema). As the disruption becomes worse from further increases in the pulmonary venous pressure, the alveoli and airways are flooded with fluid. This is accompanied by a worsening of the arterial blood gas values (i.e., lower PaO2 and possible increased partial pressure of carbon dioxide in arterial blood [PaCO2] and progressive respiratory acidemia). ADHF can manifest as pulmonary edema. This is an acute, life-threatening situation in which the lung alveoli become filled with serosanguineous fluid (Fig. 35-3, D). The most common cause of pulmonary edema is left-sided HF secondary to CAD. (Other etiologic factors for pulmonary edema are listed in Table 28-25.) Patients with ADHF can be categorized into one of four groups based on hemodynamic and clinical status: dry-warm, dry-cold, wet-warm, and wet-cold1 (Table 35-3). The most common presentation is the warm and wet patient. This patient has adequate perfusion (warm) but has volume overload (e.g., congestion, dyspnea, edema). TABLE 35-3 CLINICAL PROFILE IN ACUTE DECOMPENSATED HEART FAILURE Chronic HF is characterized as a progressive worsening of ventricular function and chronic neurohormonal activation that results in ventricular remodeling. This process involves changes in the size, shape, and mechanical performance of the ventricle. The clinical manifestations of chronic HF depend on the patient’s age, the underlying type and extent of heart disease, and which ventricle is failing to pump effectively. Table 35-4 lists the manifestations of right-sided and left-sided chronic HF. The patient with chronic HF usually has manifestations of biventricular failure. The Heart Failure Society of America (HFSA) developed the acronym FACES (Fatigue, limitation of Activities, chest Congestion/cough, Edema, and Shortness of breath) to help teach patients to identify HF symptoms7 (see eTable 35-1 on the website for this chapter). TABLE 35-4 MANIFESTATIONS OF HEART FAILURE Pleural effusion results from increasing pressure in the pleural capillaries. A transudation of fluid occurs from these capillaries into the pleural space. (Pleural effusion is discussed in Chapter 28.) In 1964 the New York Heart Association (NYHA) developed functional guidelines for classifying people with heart disease based on tolerance to physical activity. Because this system only reflected exercise capacity, the American College of Cardiology Foundation/AHA (ACCF/AHA) developed a staging system that identified disease progression and treatment strategies.1 This system allows for identification of people at risk for developing HF but who do not currently have heart disease. The ACCF/AHA system encourages clinicians to actively address the patient’s risk factors and treat any existing conditions to prevent further disease progression. This may help reduce the growing number of HF patients. The two systems are compared in Table 35-5. TABLE 35-5 NYHA FUNCTIONAL CLASSIFICATION OF HEART DISEASE AND ACCF/AHA STAGES OF HEART FAILURE Sources: The Criteria Committee of the New York Heart Association: Nomenclature and criteria for diagnosis of diseases of the heart and great vessels, ed 9, Boston, 1994, Little, Brown & Co. and Yancy CW, Jessup M, Bozkurt B, et al: 2013 ACCF/AHA guideline for the management of heart failure: executive summary, Circulation 128:e1, 2013. Diagnosing HF is often difficult, since neither patient signs nor symptoms are highly specific, and both may mimic those associated with many other medical conditions (e.g., anemia, lung disease). Diagnostic tests for ADHF and chronic HF are presented in Table 35-6. A primary goal in diagnosis is to find the underlying cause of HF. TABLE 35-6 COLLABORATIVE CARE • History and physical examination • Determination of underlying cause • Serum chemistries, cardiac markers, BNP or NT-proBNP level (see Table 32-6), liver function tests, thyroid function tests, CBC, lipid profile, kidney function tests, urinalysis • 2-dimensional echocardiogram • Nuclear imaging studies (see Table 32-6) • Circulatory assist device: intraaortic balloon pump • Endotracheal intubation and mechanical ventilation • Vital signs, urine output at least q1hr • Continuous ECG and pulse oximetry monitoring • Hemodynamic monitoring (e.g., intraarterial BP, PAWP, CO) • Drug therapy (see Table 35-7) • Possible cardioversion (e.g., atrial fibrillation) An endomyocardial biopsy (EMB) may be done in patients who develop unexplained, new-onset HF that is unresponsive to usual care.8 EF is used to differentiate systolic and diastolic HF. This distinction is important to make in the early treatment of HF. EF is measured using echocardiography and/or nuclear imaging studies (see Table 32-6). Other useful diagnostic tests include electrocardiogram (ECG), chest x-ray, and heart catheterization. Laboratory studies also aid in the diagnosis of HF. In general, BNP levels correlate positively with the degree of left ventricular failure. Many laboratories routinely measure the N-terminal prohormone of BNP (NT-proBNP). This is a more precise assay to aid in the diagnosis of HF (see Table 32-6). Levels are temporarily higher in patients receiving nesiritide (Natrecor) and may be high in patients with chronic, stable HF. Increases in BNP or NT-proBNP levels can be caused by conditions other than HF. These conditions include pulmonary embolism, renal failure, and acute coronary syndrome. With the addition of new drugs and device therapies, the management of HF has dramatically changed in the past few years. Because of the large number of patients and the high cost of care related to hospital readmissions, strategies to improve outcomes have been developed. One example is the use of guideline-directed medical therapy as defined by the AACF/AHA.1 Another example is specialized HF inpatient units with transitional programs to the outpatient setting to help manage these patients. These units are staffed with multidisciplinary HF teams, including nurses who are educated in the care of these patients. Table 35-6 lists the collaborative therapy for the patient with ADHF. Patients with ADHF need continuous monitoring and assessment. This may be done in an intensive care unit (ICU) if the patient is unstable. In the ICU, you will monitor ECG and oxygen saturation continuously. Assess vital signs and urine output at least every hour. The patient may have hemodynamic monitoring, including intraarterial BP and pulmonary artery pressures (PAPs). If a pulmonary artery catheter is placed, evaluate CO and pulmonary artery wedge pressure (PAWP). Therapy is titrated to maximize CO and reduce PAWP. A normal PAWP is generally between 8 and 12 mm Hg. Patients with ADHF may have a PAWP as high as 30 mm Hg. (Hemodynamic monitoring is discussed in Chapter 66.) Supplemental oxygen helps increase the percentage of oxygen in inspired air. (Oxygen therapy is discussed in Chapter 29.) In severe pulmonary edema the patient may need noninvasive ventilatory support (e.g., bilevel positive airway pressure [BiPAP]) or intubation and mechanical ventilation. BiPAP is also effective in decreasing preload. (Ventilatory support is discussed in Chapter 66.) Ultrafiltration (UF), or aquapheresis, is an option for the patient with volume overload.9 It is a process to remove excess salt and water from the patient’s blood. UF can rapidly remove intravascular fluid volume while maintaining hemodynamic stability. The ideal patients for UF are those with major pulmonary or systemic volume overload who have shown resistance to diuretics and are hemodynamically stable. UF also may be an appropriate adjunctive therapy for patients with HF and coexisting renal failure. (UF is discussed in Chapter 47.) Once the patient is more stable, determination of the cause of ADHF and pulmonary edema is important. Diagnosis of systolic or diastolic HF will then direct further management protocols. Circulatory assist devices are used to manage patients with worsening HF. The intraaortic balloon pump (IABP) is a device that increases coronary blood flow to the heart muscle and decreases the heart’s workload through a process called counterpulsation. It is useful in hemodynamically unstable patients because it decreases SVR, PAWP, and PAP, leading to improved CO. Ventricular assist devices (VADs) can be used to maintain the pumping action of a heart that cannot effectively contract by itself. A VAD is a battery-operated, mechanical pump that is surgically implanted. (IABPs and VADs are discussed in Chapter 66.) Coexisting psychologic disorders, especially depression and anxiety, contribute to an increased risk of mortality and higher readmission rates and health care costs in patients with HF.10 In addition, patients with psychologic disorders have poorer adherence to treatment and self-care.11 Assess patients with HF for depression and anxiety and, if appropriate, initiate treatment plans. Drug therapy is essential in treating ADHF (Table 35-7). TABLE 35-7
Nursing Management
Heart Failure
Heart Failure
Etiology and Pathophysiology

Cause
Mechanism
Anemia
↓ O2-carrying capacity of the blood stimulating ↑ in CO to meet tissue demands, leading to increase in cardiac workload and increase in size of LV
Infection
↑ O2 demand of tissues, stimulating ↑ CO
Thyrotoxicosis
Changes the tissue metabolic rate, ↑ HR and workload of the heart
Hypothyroidism
Indirectly predisposes to ↑ atherosclerosis; severe hypothyroidism decreases myocardial contractility
Dysrhythmias
May ↓ CO and ↑ workload and O2 requirements of myocardial tissue
Bacterial endocarditis
Infection: ↑ metabolic demands and O2 requirements
Valvular dysfunction: causes stenosis and regurgitation
Pulmonary embolism
↑ Pulmonary pressure resulting from obstruction leads to pulmonary hypertension, ↓ CO
Paget‘s disease
↑ Workload of the heart by ↑ vascular bed in the skeletal muscle
Nutritional deficiencies
May ↓ cardiac function by ↑ myocardial muscle mass and myocardial contractility
Hypervolemia
↑ Preload causing volume overload on the RV
![]() Genetic Link
Genetic Link
Pathophysiology of Ventricular Failure.
Diastolic Failure.
Mixed Systolic and Diastolic Failure.
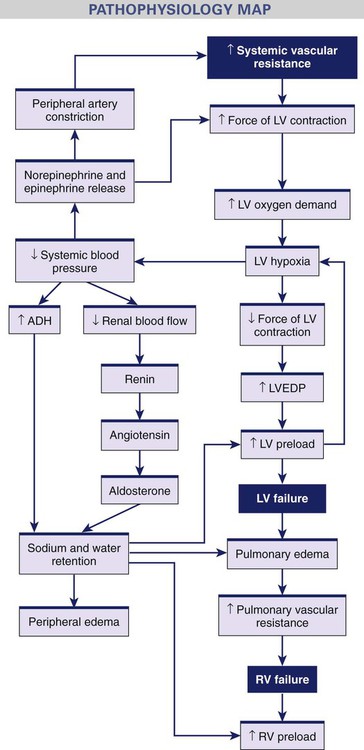
Compensatory Mechanisms.
Neurohormonal Response.
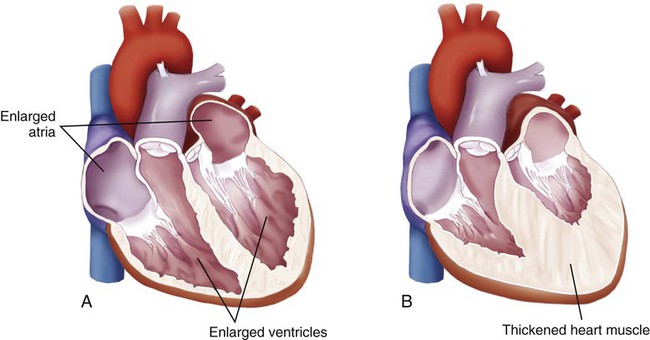
Dilation.
Hypertrophy.
Counterregulatory Mechanisms.
Types of Heart Failure
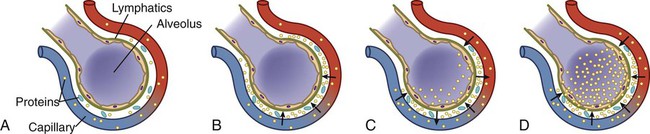
Clinical Manifestations Acute Decompensated Heart Failure
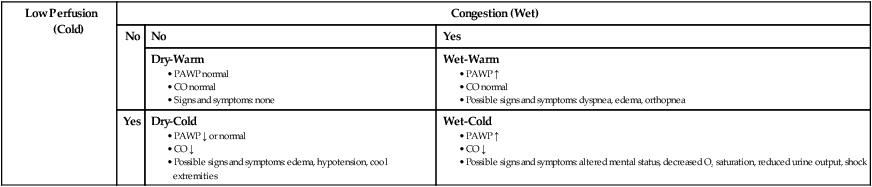
Low Perfusion (Cold)
Congestion (Wet)
No
No
Yes
Dry-Warm
Wet-Warm
Yes
Dry-Cold
Wet-Cold
Clinical Manifestations Chronic Heart Failure
Right-Sided Heart Failure
Left-Sided Heart Failure
Signs
Symptoms
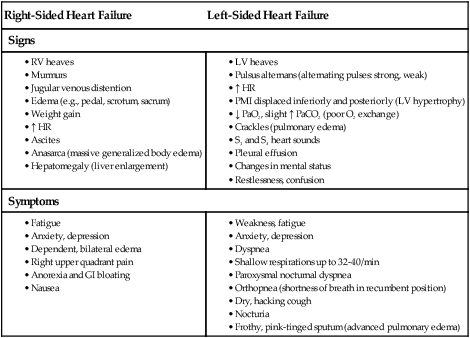
Complications of Heart Failure
Pleural Effusion.
Classification of Heart Failure
NYHA Functional Classification of Heart Disease
ACCF/AHA Stages of Heart Failure
Class I
Stage A
No limitation of physical activity. Ordinary physical activity does not cause fatigue, dyspnea, palpitations, or anginal pain.
Class II
Slight limitation of physical activity. No symptoms at rest. Ordinary physical activity results in fatigue, dyspnea, palpitations, or anginal pain.
Class III
Marked limitation of physical activity but usually comfortable at rest. Less than ordinary physical activity causes fatigue, dyspnea, palpitations, or anginal pain.
Class IV
Inability to carry on any physical activity without discomfort. Symptoms of cardiac insufficiency or of angina may be present even at rest. If any physical activity is undertaken, discomfort is increased.
Patients at high risk for HF (e.g., patients with hypertension, diabetes, metabolic syndrome) but without structural heart disease or symptoms of HF.
Stage B
Patients with structural heart disease (e.g., patients with history of MI, valve disease) but who have never shown signs or symptoms of HF.
Stage C
Patients with prior or current symptoms of HF associated with known, underlying structural heart disease.
Stage D
Patients with refractory HF (e.g., patients with severe symptoms at rest despite maximal medical therapy) who require specialized interventions.
Diagnostic Studies
Heart Failure
Both ADHF and Chronic HF
ADHF
Chronic HF
Diagnostic
Collaborative Therapy
Collaborative Care Acute Decompensated Heart Failure
Drug Therapy.
Drug
Mechanism of Action
Diuretics (see Table 33-7)
Loop Diuretics
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

Nursing Management: Heart Failure
Get Clinical Tree app for offline access