Microbiology
Objectives
After completing this chapter you should be able to:
Fundamental Concepts
3. Describe the proper collection and transportation procedures for microbiological specimens.
4. Explain the importance of Gram staining in microbiology.
7. Describe the procedures for acid-fast stains, wet mounts, and KOH preparation.
8. Explain the cellulose tape procedure for the identification of pinworms.
CLIA-Waived Microbiology Tests
Advanced Concepts
1. List and describe the types of culture media and their uses.
3. Discuss culture plate streaking methods for colony isolation and colony counting.
4. Explain the function of sensitivity testing.
5. List pathogenic bacteria, fungi, and parasites frequently seen in the physician’s office laboratory.
6. List some of the emerging infectious diseases.
7. Discuss some of the organisms that could be used in bioterrorism.
Key Terms
aerobic requiring oxygen for growth
aerosols fine particles suspended in air
anaerobic able to grow and function in the absence of oxygen
binary fission asexual reproduction in which the cell splits in half
capnophilic requiring some carbon dioxide to grow
colony visible mass of bacteria formed on a culture medium by one bacterium growing and replicating
culture the reproduction of microorganisms in a laboratory culture medium
eukaryotic pertaining to organisms that possess a true nucleus with a nuclear membrane and organelles
expectoration coughing up of sputum and mucus from the trachea and lungs
facultative anaerobe organism that grows with or without oxygen
fastidious requiring special nutrients or conditions for growth
gram-negative having the pink/red color of the counterstain used in Gram’s method of staining microorganisms
gram-positive retaining the purple color of the stain used in Gram’s method of staining microorganisms
hyphae tubelike filaments
inoculation process of transferring microorganisms into or on a culture medium for growth
malaise feeling of weakness, distress, or discomfort
media (singular: medium) liquid, semisolid, or solid substances containing nutrients needed to grow microorganisms
microaerophilic requiring reduced oxygen for growth
microbiology study of microorganisms, including bacteria, fungi, protozoa, and viruses
microorganism any tiny, usually microscopic entity capable of carrying on living processes
morbidity the rate at which an illness occurs
mortality the rate of deaths
myalgia diffuse muscle pain
mycelium mass of hyphae that some fungi produce
Petri dish dish containing medium in which to grow microorganisms
peptidoglycan component made of polysaccharides and peptides that gives rigidity to the bacterial cell wall
prokaryotic pertaining to unicellular organisms that do not have a true nucleus with a nuclear membrane
 Fundamental Concepts
Fundamental Concepts
Overview of Microbiology
Microbiology is the study of microorganisms, including bacteria, fungi, protozoa, and viruses. According to Mosby’s Medical Dictionary, a microorganism is “any tiny, usually microscopic entity capable of carrying on living processes.” Microorganisms were first discovered in 1676, when a Dutch linen merchant and self-made microbiologist named Anthony van Leeuwenhoek created a magnifying glass through which they could be observed.
Microorganisms are found everywhere—in the air, soil, and water. Some microorganisms are involved in the decomposition of waste and the natural recycling process of life and death. Microorganisms that are normally found in and on the human body are referred to as normal flora or normal biota. Normal flora assist in preventing the growth and spread of disease-causing microorganisms. An example of protective normal flora is the bacteria Lactobacillus, which creates an acidic environment in the female vagina. Certain antibiotics destroy Lactobacillus, thereby allowing opportunistic infections such as candidiasis (caused by the yeast Candida albicans) to take hold.
Research has determined that fewer than 1% of all microorganisms are pathogens. When pathogenic microorganisms invade the body and overcome its natural defense mechanisms, as discussed in Chapter 7, an infection occurs. Some infections are contagious, which means they can spread from person to person.
The role that pathogenic microorganisms play in causing disease should be understood, as well as the means to prevent the spread of these organisms. Table 8-1 shows a timeline of efforts to control infectious diseases throughout history.
TABLE 8-1

When a patient contracts an infection, the first step is to identify the pathogenic organism that is causing the problem, then find the appropriate treatment to bring the infection under control. Following is an overview of how microorganisms are classified.
Classification of Microorganisms
Although the more complex microorganism identification procedures are not performed in all laboratories, it is important to have a general knowledge of the classification, nomenclature, structure, and growth requirements of pathogenic microorganisms. Microorganisms are categorized as viruses, bacteria, fungi, or parasites.
Viruses
Viruses are the smallest microorganisms, consisting of either ribonucleic acid (RNA) or deoxyribonucleic acid (DNA) molecules. They are so small they can be seen only under a very high magnification using an electron microscope. They need to live in a host cell and use the host cell’s metabolic machinery to multiply. Therefore, unlike bacteria and other microorganisms, viruses must be grown inside tissue cells rather than on typical culture media (liquid, semisolid, or solid substances containing nutrients needed to grow microorganisms).
Bacteria
Bacteria are classified as prokaryotic, which means they are unicellular organisms that do not have a true nucleus with a nuclear membrane. Bacteria do have a cell membrane and a cell wall. They reproduce by binary fission, asexual reproduction in which the cell splits in half. Bacteria can reproduce very rapidly on the culture media containing nutrients for their growth. They are able to form colonies (visible masses of bacteria formed on a culture medium by one bacterium growing and replicating itself). Culture is the reproduction of microorganisms in a laboratory culture medium.
Fungi
Fungi (singular: fungus) are eukaryotic, which means they possess a true nucleus with a nuclear membrane. They lack chlorophyll, can be grown on media, and are generally classified as molds or yeasts. Yeasts are single cells that reproduce by budding, in which a new cell forms on the surface of the yeast, grows, pinches off, and becomes a separate cell. They form colonies with a smooth, creamy appearance on culture media. In contrast, molds are made up of mycelium, which consists of tubelike structures called hyphae, and have a fuzzy or woolly appearance.
Parasites
Parasites are organisms that live in or on a host and derive nourishment from the host. They can be one-celled protozoa (e.g., amoeba) or many-celled organisms such as helminths (roundworms, tapeworms, and flukes).
Nomenclature of Microorganisms
More than 250 years ago Carl von Linné, a Swedish botanist, created a nomenclature system for all living things. In this system each plant and animal has a name with two parts: genus (group) and species (kind). The standard rule is to capitalize the genus name and write the species name in lowercase. The genus and species names are always underlined or italicized, as in Staphylococcus aureus.
Structural Characteristics of Bacteria
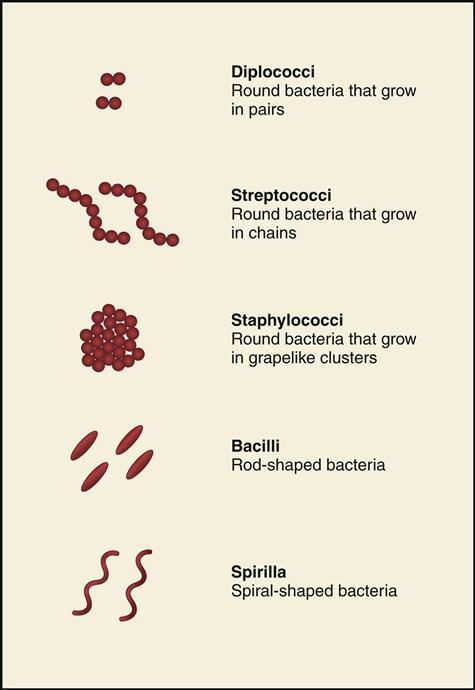
Bacteria are classified and identified according to their shapes and growing patterns. Fig. 8-1 illustrates three growing patterns of the circular cocci (diplococci, streptococci, and staphylococci), as well as rod-shaped bacilli and spiral-shaped spirilla.
Coccus
The coccus (plural: cocci) is a round bacteria that grows in a variety of formations, depending on the division pattern of the cocci and whether the cocci remain attached afterwards. Cocci can be found as singles, pairs (diplococci), tetrads (patterns of four), chains (e.g., Streptococcus), clusters (e.g., Staphylococcus, seen as grapelike clusters).
The most common species of Staphylococci are epidermidis and aureus. Staphylococcus epidermidis is found normally on the surface of the skin and the mucous membrane of the mouth. S. aureus, on the other hand, is commonly associated with pathogenic conditions such as boils, carbuncles, pimples, impetigo, abscesses, wound infections, and even food poisoning.
Streptococci are cocci arranged in chains. Although some Streptococci are part of normal flora, some cause pathogenic conditions such as strep throat, scarlet fever, and rheumatic fever, which are discussed later in this chapter. In addition, Streptococcus can cause carbuncles, impetigo, pneumonia, puerperal sepsis (infection after childbirth), and erysipelas (infectious skin disease).
Bacillus
The bacillus (plural: bacilli) is rod shaped and can also be described as coccobacillus when it appears as a combination of cocci and bacilli (rod-shaped but rounded on the ends) or diplobacillus (when it is arranged in pairs).
Bacilli cause diseases such as urinary tract infections, botulism, tetanus, gas gangrene, gastroenteritis, typhoid fever, pertussis (whooping cough), bacillary dysentery, diphtheria, tuberculosis, leprosy, and the plague.
Spirillum
Spirillum (plural: spirilla) is a spiral-shaped microorganism that can be a gently curved rod or look more like a corkscrew or a spring. Some spiral bacteria are Treponema pallidum, which causes syphilis, and Vibrio cholerae, which causes cholera.
Other Bacterial Structures
Some bacteria have the following additional structures:
Collecting, Transporting, and Processing Microbiology Specimens
Collection of microbiological specimens requires the use of infection control techniques and precautions to prevent infecting yourself or others. The following precautions should be observed at all times:
• Wear the appropriate personal protection devices.
• Disinfect specimen containers after collection.
• Put the specimens in transport bags after collection.
As with all specimen collections, the results are only as good as the specimen collected. The ideal time to collect a microbiologic specimen is before antibiotics have been administered because the antibiotics will kill the microbes that are needed to be grown and identified.
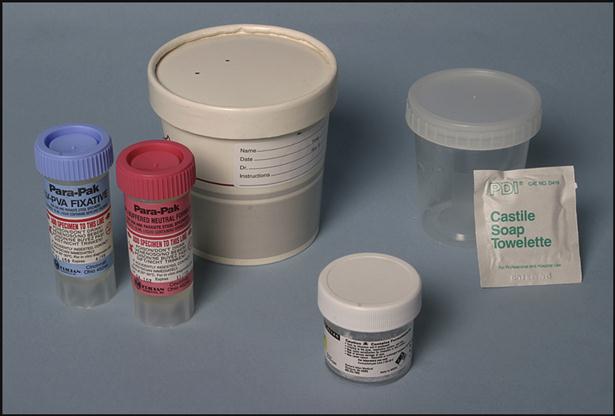
Specific containers are used to transport a variety of specimens, such as blood cultures, body fluids, skin scrapings, exudates from deep wounds or abscesses, sputum from the lungs, and feces and urine specimens (Fig. 8-2). The specific directions for collecting these specimens will be discussed later in the chapter.
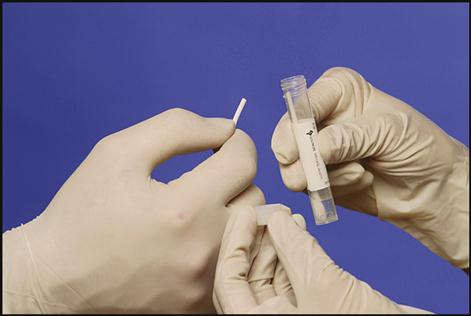
Collecting a microbiological specimen from the following sites generally involves applying a swab to the infected area: eyes, rectum, urogenital areas, throat, and wounds. The microbiology specimens must be processed as soon as possible. Transport systems provide the conditions microorganisms need to survive during the transport to the laboratory. These transport systems contain maintenance media that prevent the microorganisms from dying or multiplying. Two examples of transport systems are shown in Fig. 8-3. The system at the top of the picture includes a blue-handled Dacron swab on a plastic stick. When the specimen is obtained, the swab is put into the holder and the bottom of the tube is squeezed, releasing the transport media onto the swab to maintain the organisms. The swab with the purple handle, at the bottom of the picture, contains dried nutrient media. The transport system to be used will depend on the type of specimen collected or the specific organisms for which the culture was ordered.
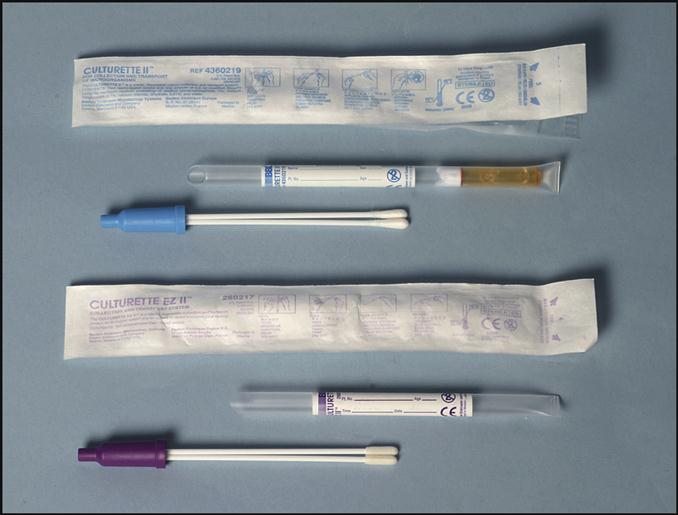
Collecting a Throat Specimen
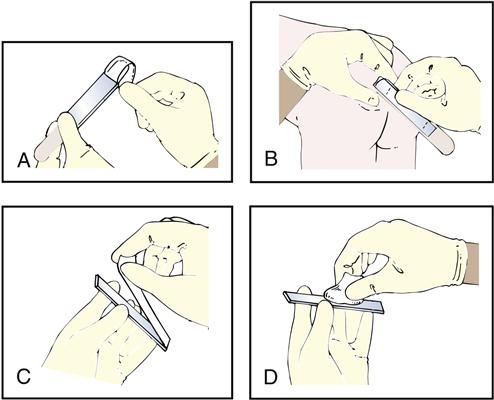
The swabbed throat specimen collection is one of the most common microbiology procedures performed in the physician’s office laboratory (POL). The specimen is tested for possible streptococcal infections.
The Collecting a Throat Specimen procedure check sheet is provided in the workbook and is demonstrated in Procedure 8-1, located at the end of this section.
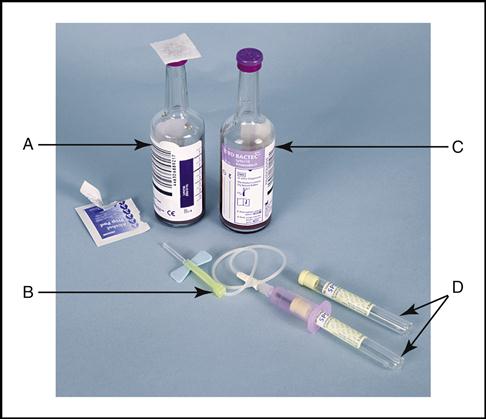
Collecting a Blood Culture Specimen
Blood cultures are collected in containers such as BACTEC bottles (Becton Dickinson, Franklin Lakes, NJ) (Fig. 8-4). Approximately 10 mL of blood is required for each bottle when collecting blood from an adult. (NOTE: The aerobic bottle grows oxygen-dependent bacteria and must be drawn first when using the butterfly method of draw, and the anaerobic bottle grows bacteria that grow best in the absence of oxygen. It is drawn second. Also displayed are the Vacutainer tubes with their light yellow caps.) Pediatric patients generally need 1 to 3 mL for one aerobic bottle or the culture tube. When a patient has a fever of unknown origin, the physician often orders a blood culture to determine whether microorganisms are present in the blood. A specific aseptic preparation of the puncture site and the equipment is required when drawing blood cultures. The anaerobic bottle is filled first unless the butterfly method is used, in which case the aerobic bottle is filled first because of the air in the tubing.
Collecting a Urine Culture Specimen
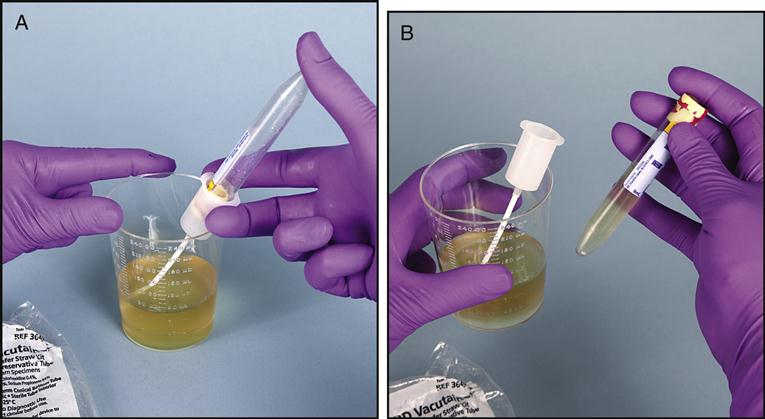
When a urinary tract infection is present, the physician will typically order a urine culture and sensitivity to determine the type of pathogen causing the infection and the antibiotic that will most effectively treat the infection. The patient is instructed to collect a clean-catch midstream urine into a sterile specimen container, as described in Chapter 3. If the urine specimen must be transported to the microbiology lab, the urine can be transferred into a sterile Vacutainer tube with the appropriate preservatives, as seen in Fig. 8-5, A and B.
Collecting a Chlamydia Specimen
Another type of transport media is that used for Chlamydia organisms (Fig. 8-6). Chlamydia is a sexually transmitted disease caused by Chlamydia trachomatis, a tiny gram-negative (having the pink/red color of the counterstain used in Gram’s method of staining microorganisms) intracellular bacterium that requires a host cell for growth. Women with this disease may have no symptoms or may have dysuria; itching; irritation of the genital area; and an odorless, yellowish vaginal discharge. Left untreated, chlamydia can cause pelvic inflammatory disease and infertility. The symptoms in men are mild dysuria and a thin, watery discharge from the penis. If not treated in men, the disease can cause epididymitis, which could result in infertility. Using a sterile swab, the physician collects a specimen from the female endocervical canal or the male urethra. The swab is placed in a tube containing a transport medium to preserve the specimen and is sent to a reference laboratory for testing. Testing for Chlamydia is most frequently done using a DNA-probe test (test based on DNA detection).
Collecting a Gonorrhea Specimen
The JEMBEC transport system (Becton Dickinson) (Fig. 8-7) consists of a JEMBEC plate, a small white pill, and a plastic bag. It is used for transporting and growing Neisseria gonorrhoeae, the gram-negative diplococcus that causes gonorrhea. When activated, the pill creates a 10% carbon dioxide atmosphere that this organism needs for growth. Gonorrhea is a sexually transmitted disease of the genitourinary tract. Women either show no symptoms or have dysuria and a yellow discharge. If untreated, the infection can result in pelvic inflammatory disease, which could lead to infertility. Men infected with gonorrhea have dysuria and may have a whitish discharge from the penis that may become thick and creamy. In untreated men epididymitis may occur, which can lead to infertility. Like Chlamydia, N. gonorrhoeae can be diagnosed with a DNA probe, which tests for the presence of the gonorrhea gene. The collection procedure is the same as for Chlamydia.
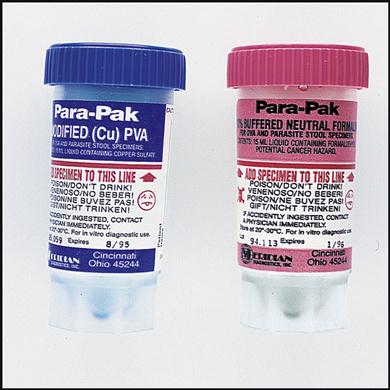
Collecting a Fecal Specimen for Ova and Parasites
Another transport method is ova and parasite collection kits, which consist of two containers, one with formalin and one with polyvinyl alcohol (Fig. 8-8). The containers must be filled to the appropriate line with a fecal specimen. The kit is used for the preservation of parasites.
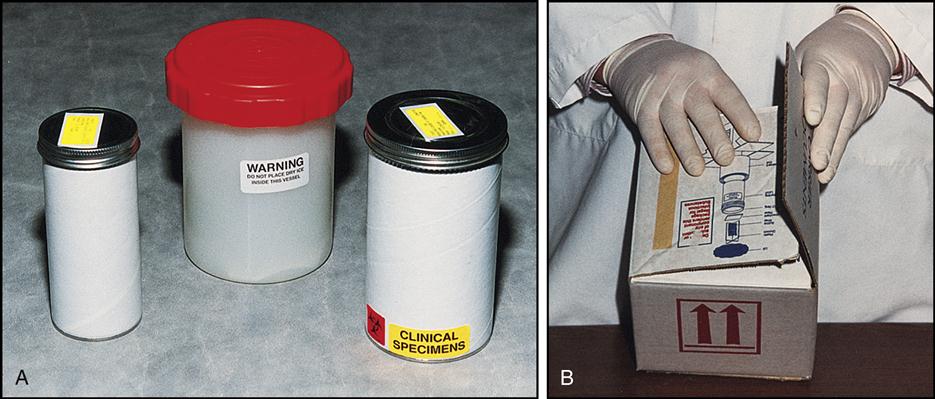
Transporting Specimens by Mail
When specimens are transported by mail, they must be packed in the correct container (Fig. 8-9). The specimen is placed in a container, which is then placed inside a mailing container. A biohazard emblem must be affixed to every container, including the outside one.
Microbiology Smears, Stains, and Wet Mounts
After the microbiology specimen is collected, the next step is to identify the infectious microorganism microscopically. A portion of the specimen is generally smeared onto a slide or placed into a solution on a slide. The smeared specimen is then fixed to the slide and stained to demonstrate its staining characteristics.
Gram Stain
More than 100 years have passed since the Gram stain was developed by Dr. Hans Christian Gram. Gram stain is defined by Mosby’s Medical Dictionary as a “method of staining microorganisms…that serves as a primary means of identifying and classifying bacteria.” The Gram stain reaction is still used today to distinguish between gram-positive (retaining the purple color of the stain) and gram-negative organisms. Table 8-2 lists the four reagents used in Gram staining and the positive (purple) and negative (pink/red) results that can occur.
TABLE 8-2
| Stain Step | Staining Reagent | Color of Gram-Positive Organism | Example Gram-Positive Cocci (GPC) | Color of Gram-Negative | Example Gram-Negative Bacilli (GNB) |
| 1. Primary stain | Crystal violet | Purple |  | Purple |  |
| 2. Mordant | Gram’s iodine | Purple |  | Purple |  |
| 3. Decolorizer | Acetone or ethyl alcohol | Purple |  | Colorless |  |
| 4. Counterstain | Safranin | Purple |  | Pink/red |  |
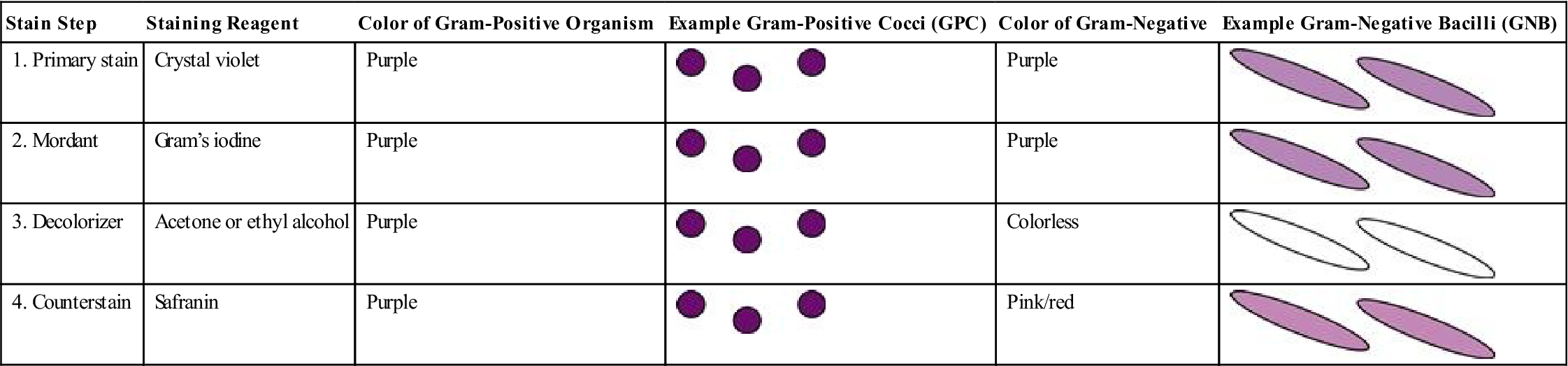
Medical assistants should be able to perform a Gram stain on a specimen that has been smeared and fixed on a slide. Although medical assistants will not interpret Gram stains, it is important that they understand why the identification of bacterial shapes and growth patterns is important, as well as understand the significance of whether an organism is gram positive (purple) or gram negative (pink/red). (See Tables 8-4 and 8-5 in the Advanced Concepts section of this chapter for the lists of pathogenic bacilli and cocci, with their Gram reactions in Column 3 of the tables.)
Smear Preparation
The bacterial specimen must be smeared onto a glass slide, fixed, and then stained to identify it as gram positive or negative. The specimen is obtained from the following three possible sources:
The smear is allowed to dry and is then heat fixed either by running it through a natural gas Bunsen burner or by placing the slide on an electric incinerator for a few seconds (do not overheat because this could cause distortion of the bacterial cells). Heat fixing kills the microorganisms and causes the swabbed material to stick to the slide before staining. The methanol fixative used in blood smears may also be used to affix the smear to the slide.
The most critical step in the Gram stain procedure is the decolorizing step. If the decolorizing is not done for a sufficient length of time, everything will be purple, and, conversely, if decolorizing is done for too long a duration, everything will be pink/red.
Gram-positive organisms stain purple because a large peptidoglycan layer is present in their cell walls that holds the crystal violet stain. Peptidoglycan is a component made of polysaccharides and peptides that gives rigidity to the bacteria’s cell walls. Gram-negative organisms, on the other hand, have a small layer of peptidoglycan. In addition, they have an outer layer of lipoprotein and lipopolysaccharides (lipids) that are soluble in the decolorizing solution. When the decolorizer is applied, it punches holes in the lipid portion of the gram-negative outer layer, allowing the crystal violet to seep out. A counterstain of safranin stains all the material that is not stained by the crystal violet. At the end of the staining procedure, the gram-positive organisms are purple and the gram-negative organisms are pink/red.
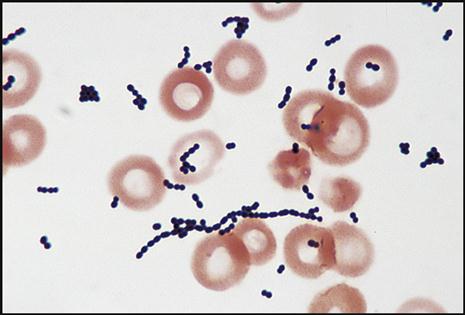
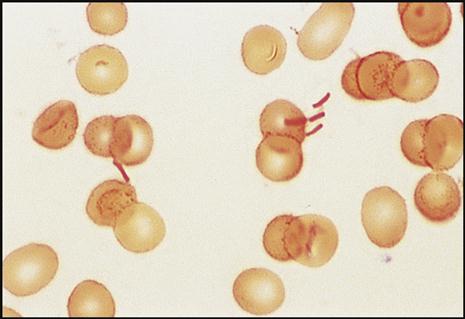
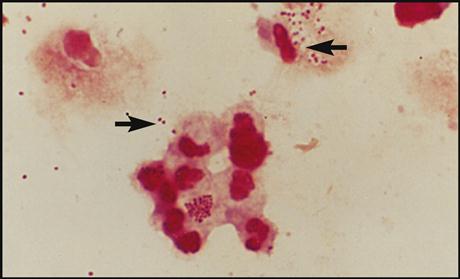
The gram reaction and shape of the pathogen give the microbiologist the necessary information to help identify the bacteria that are causing the infection in question. For example, gram-positive cocci (GPC) are seen as purple, circular bacteria (Fig. 8-10), and gram-negative bacilli (GNB) are seen as pink/red, rod-shaped bacteria (Fig. 8-11). N. gonorrhoeae (Fig. 8-12) are gram-negative cocci (GNC) that appear as pairs of pink/red diplococci shaped like kidney beans and facing each other.
The Gram stain procedure check sheet is provided in the workbook and is demonstrated in Procedure 8-2, located at the end of this section.
Acid-Fast Stains
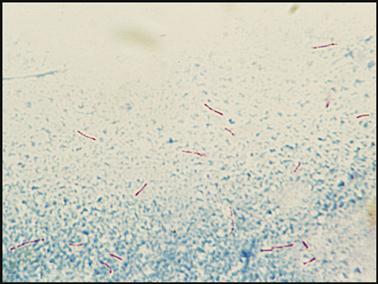
The acid-fast stain is used to microscopically search for microorganisms in the Mycobacterium genus referred to as acid-fast bacilli (AFB), which resist decolorization with acid alcohol. Mycobacterium tuberculosis causes tuberculosis (TB), a lung infection in which tubercles (nodules) are formed. Symptoms include night sweats, pulmonary hemorrhage, and expectoration of purulent sputum. Another species, Mycobacterium avium complex, disseminates throughout the body in patients with acquired immune-deficiency syndrome (AIDS). Both mycobacterial diseases have been on the increase with the spread of AIDS.
Although AFB staining is not done in a POL, medical assistants should understand how the stain is done and recognize the appearance of the stained AFB organism. Two methods of AFB staining break down the wax in the AFB cell wall: the Ziehl–Neelsen stain, which uses heat, and Kinyoun, a cold stain that mixes a detergent with the dye. Both methods use carbolfuchsin solutions for the primary stain, decolorize with acid alcohol, and counterstain with methylene blue. When the stain is complete, the acid-fast organisms appear as red beaded rods (Fig. 8-13). The slide is observed under the oil immersion objective.
Fluorescent staining is another method. Auramine or auramine–rhodamine fluorochrome stain is used. It is viewed under a fluorescence microscope equipped with the appropriate filter system for this type of stained smear. The stain may be screened at a lower magnification, which allows more fields to be examined in a shorter period of time. TB organisms appear as bright yellow-orange bacilli against a dark background.
Wet Mounts
Wet mounts are done to view organisms in their living state. One type of wet mount determines the motility of a parasite, Trichomonas vaginalis (Fig. 8-14). This organism is a pear-shaped protozoa with four flagella that give it a characteristic jerky movement. T. vaginalis is one of the most common sexually transmitted diseases. In women the infection is located primarily in the vagina, where it causes itching and a frothy, creamy discharge. Men are usually asymptomatic and serve as carriers. The organism is found in urine or vaginal specimens in women and urine or prostatic sections in men.
Wet mounts are also done to detect the presence of “clue cells,” vaginal epithelial cells covered with Gardnerella vaginalis, a gram-negative coccobacillus. This organism is found in almost 100% of women who have bacterial vaginosis. Infected women produce a watery exudate that lacks white blood cells and usually has a fishy odor, and the clue cells are covered by the Gardnerella organism.
The Wet Mount procedure check sheet is provided in the workbook and is demonstrated in Procedure 8-3, located at the end of this section.
KOH Preparation
Another type of microbiology slide identification is the KOH (potassium hydroxide) preparation. KOH is an alkaline solution that breaks down protein material, facilitating the identification of any fungus that may be present in skin scrapings or mucus. A KOH preparation shows fungal hyphae from skin dermatophyte infections, as well as yeast such as C. albicans. Although the KOH preparation may be done by a medical assistant, it is interpreted by a trained laboratorian or physician.
The KOH Preparation procedure check sheet is provided in the workbook and is demonstrated in Procedure 8-4, located at the end of this section.
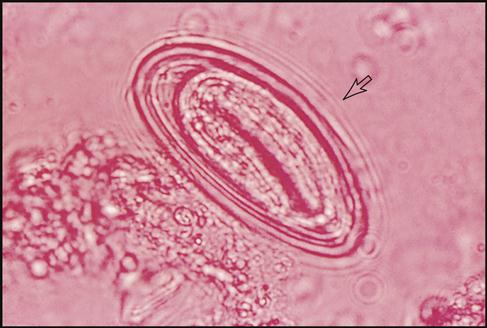
Pinworm Specimen Collection and Microscopic Results
Enterobius vermicularis is a tiny roundworm known commonly as pinworm. The female pinworm normally lays her eggs during the nighttime hours in the anal area of the human host. This causes itching, and the host scratches this area, infecting the hands and fingernails. The identification test is performed by using either swabs coated with petroleum jelly or cellulose tape.
The cellulose tape test consists of placing cellulose tape (sticky side) over the patient’s anal area and then placing the tape sticky-side-down on a glass slide (Fig. 8-15). A physician or trained laboratorian examines the tape microscopically for E. vermicularis eggs (Fig. 8-16).