Chapter 46 Neonatal jaundice
Introduction
A significant number of babies with acute bilirubin encephalopathy and subsequent kernicterus are being reported (Manning et al 2007). This may further increase as mothers and babies are discharged home earlier before feeding is established, especially if community services are not in place to support a policy of early discharge (Sellwood & Huertas-Ceballos 2008).
Physiology
The majority of bilirubin forms from the breakdown of heme, an iron-containing molecule, an essential component of cytochromes, myoglobin and haemoglobin. At the end of their lifespan, red blood cells are sequestered by the spleen and the haemoglobin is broken down into component parts of heme and globin. The iron molecule is then removed from the heme to be recycled and the heme molecule is oxidized to biliverdin, which is then reduced to form unconjugated bilirubin (Dennery et al 2001). Increased red cell breakdown leads to increased levels of unconjugated bilirubin. Bilirubin is a lipid-soluble molecule that easily crosses lipid membranes, such as those within the brain. The insolubility in water means that bilirubin must be transported in the bloodstream linked to albumin. In this protein-bound state, bilirubin is not available to be filtered by the glomerulus or to enter into cell tissues (see website).
Physiological jaundice
Features of physiological jaundice
Supplementing breastfeeding with water or glucose appears to have no effect on bilirubin levels in healthy newborns (Nicoll et al 1982) and should be avoided. Physiological jaundice may be exacerbated in situations that lead to increased bilirubin production (for example, polycythaemia, bruising) or decreased bilirubin excretion (poor feeding with delayed intestinal transit). In the past it was believed that physiological jaundice could not lead to kernicterus but unfortunately this may not be the case and vigilance is crucial.
Evaluation of jaundice
The practitioner needs as much information as possible to accurately assess the risk of the baby developing jaundice, its significance and management plan. The maternal blood group should be known as well as any familial predisposition to neonatal jaundice. Risk factors include infection during pregnancy and delivery, and any bruising or perinatal trauma to the baby. Babies at high risk of developing jaundice may need to remain under hospital care for longer and research continues into accurately identifying babies at risk (Sanpavat et al 2005).
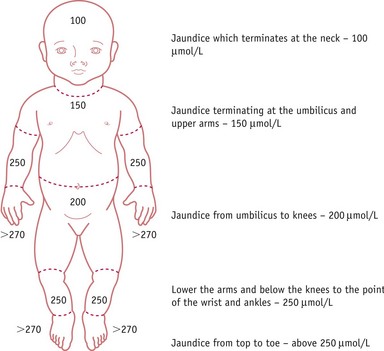
Jaundice progresses from head to toe and resolves in the opposite direction, from toe to head, therefore observation of the colour of the sclera is not useful in assessing improvement. Clinical assessment of severity by experienced practitioners can be highly accurate (Riskin et al 2003), though caution is required in babies with dark skin where visual estimation alone may lead to error (AAP 2004, NICE 2010).
Clinical evaluation of jaundice requires that the baby is undressed, and cephalocaudal progression of jaundice evaluated using the five zones as in Figure 46.1. The Kramer (1969) tool facilitates assessment of the advancement of dermal icterus, and if the baby rapidly advances down the scale, swift referral to the neonatologist for diagnosis and treatment can be achieved. Jaundice becomes clinically apparent when the serum bilirubin rises above 85 µmol/L (bilirubin can be measured as a concentration recorded in µmol/L or mg/dL). Any baby with significant jaundice at 48 hours should have a further assessment by a health professional. Transcutaneous bilirubinometer devices have been developed that correlate well with serum bilirubin measurements, which may reduce the number of blood tests required (Briscoe et al 2002, NICE 2010, Rubaltelli et al 2001, Wong et al 2002) (Box 46.1; also see website). These devices should be used in infants over 35 weeks’ gestation and a postnatal age of greater than 24 hours, and a reading greater than 250 µmol/L is an indication for serum bilirubin testing.
Records
To facilitate effective management, bilirubin results should be sequentially recorded. Graphs are available for preterm and term babies, allowing bilirubin levels to be plotted against the time the blood sample was taken, enabling the measurement to be plotted against the baby’s age in hours (AAP 2004). This helps inform the practitioner at which level phototherapy should be commenced and when exchange transfusion may be indicated. This is crucial for babies with an unconjugated hyperbilirubinaemia. In these babies, a urine dipstick will also show a positive urobilinogen (secondary to normal enterohepatic circulation of urobilinogen) and a negative bilirubin result (conjugated bilirubin will have been excreted via the liver and bowel). The National Institute for Health and Clinical Excellence (NICE) has also developed the Bili-Wheel, which measures the bilirubin level alongside the gestation to highlight interventions required (NICE 2010) (see website).
Unconjugated hyperbilirubinaemia
Unconjugated hyperbilirubinaemia has three main causes:
Increased red cell breakdown
Haemolytic disease of the newborn
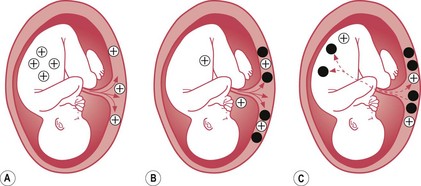
Haemolytic disease of the newborn is the immune-mediated red cell breakdown which occurs in rhesus disease and ABO incompatibility (not to be confused with haemorrhagic disease of the newborn – vitamin K deficiency). In haemolytic disease of the newborn, the maternal immune system has been ‘immunized’ against aspects of the baby’s blood group (see Fig. 46.2). This ‘immunization’ usually occurs because of a previous pregnancy, miscarriage, or following blood transfusion when fetal blood cells have passed to the maternal circulation.
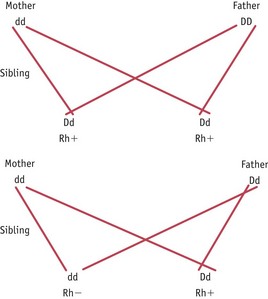
Rhesus factor is the rhesus C, D and E antigens expressed on red blood cells. It is the D antigen that is most likely to cause isoimmunization. An individual who is ‘rhesus negative’ does not express the D antigen and has the genotype dd. An individual who is ‘rhesus positive’ does express the D antigen and can be heterozygous (Dd) or homozygous (DD) for the rhesus antigen D genes. Isoimmunization in a rhesus-negative mother can occur with a heterozygous or homozygous fetus or baby and the risk of this occurring can be predicted from a knowledge of the father’s genotype as shown in Figure 46.3.
In order to prevent isoimmunization, anti-D immunoglobulin is administered to women at risk, antenatally and/or postnatally forming complexes with the fetal red cells to prevent the women’s immune system from mounting its own immune response. Current advice from NICE is to routinely administer anti-D prophylaxis to all rhesus-negative women antenatally at least once at 28 weeks’ gestation (exact regimen depends on dose used) (NICE 2008). It can be administered at times when the women are at increased risk of isoimmunization, such as after miscarriage or after the birth of a rhesus-positive baby. Women and babies at risk can be identified by taking cord and maternal blood after delivery to determine the baby’s blood group, and measure the presence of fetal blood cells and antibodies in the maternal system, as in Box 46.2.
Box 46.2
Coombs and Kleihauer tests
Anti-D is a blood product; therefore, prior to administration, informed consent must be obtained. The anti-D must be prescribed on the woman’s drug chart by a medical practitioner (NMC 2008).