Define a prototype drug.
 Distinguish between generic and trade names of drugs.
Distinguish between generic and trade names of drugs.
 Describe the main categories of controlled substances in relation to therapeutic use, potential for abuse, and regulatory requirements.
Describe the main categories of controlled substances in relation to therapeutic use, potential for abuse, and regulatory requirements.
 Identify the multiple safeguards that are in place to promote drug safety in packaging, drug laws, and approval processes.
Identify the multiple safeguards that are in place to promote drug safety in packaging, drug laws, and approval processes.
 Recognize initiatives designated to enhance safe drug administration.
Recognize initiatives designated to enhance safe drug administration.
 Develop personal techniques for learning about drugs and using drug knowledge in patient care.
Develop personal techniques for learning about drugs and using drug knowledge in patient care.
 Identify authoritative sources of drug information.
Identify authoritative sources of drug information.
Clinical Application Case Study
Joan Clark, a senior nursing student, is preparing for the NCLEX-RN examination. As she reviews material, she examines safeguards in place to protect the public from injury due to medication administration.
KEY TERMS
Biotechnology: process that may involve manipulating deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) and recombining genes into hybrid molecules that can be inserted into living organisms (often Escherichia coli bacteria) and repeatedly reproduced
Brand (trade) name: manufacturer’s chosen name for a drug, which is protected by a patent
Controlled substances: drugs that are categorized by federal law according to therapeutic usefulness and potential for abuse; also known as scheduled drugs
Drug classifications: groups of medications that are classified according to their effects on particular body systems, their therapeutic uses, and their chemical characteristics
Generic name: chemical or official name of the drug that is independent of the manufacturer and often indicates the drug group
Over-the-counter (OTC) drugs: medications available for purchase without a prescription
Pharmacoeconomics: costs of drug therapy, including costs of purchasing, dispensing, storage, administration, and laboratory and other tests used to monitor patient responses; also considers losses due to expiration
Pharmacotherapy: use of drugs to prevent, diagnose, or treat signs, symptoms, and disease processes
Placebo: inert substance containing no medication and given to reinforce a person’s expectation to improve
Prescription drugs: medications that are ordered in writing by a licensed health care provider
Prototype: often the first drug of a particular drug class to be developed; usually the standard against which newer, similar drugs are compared
Introduction
Pharmacology is the study of drugs (chemicals) that alter functions of living organisms. Pharmacotherapy, also known as drug therapy, is the use of drugs to prevent, diagnose, or treat signs, symptoms, and diseases. When prevention or cure is not a reasonable goal, relief of symptoms can greatly improve a patient’s quality of life and ability to perform activities of daily living. Contemporary nursing guidelines require that nurses keep safety issues in mind when involved in the practice of pharmacotherapy.
Drugs given for therapeutic purposes are also called medications. These substances may be given for their local or systemic effects. Drugs with local effects, such as sunscreen lotions and local anesthetics, act mainly at the site of application. Those with systemic effects are taken into the body, circulated through the bloodstream to their sites of action in various body tissues, and eventually eliminated from the body. Most drugs are given for their systemic effects. Drugs may also be given for acute disorders, such as pain or infection, or to relieve signs and symptoms of long-term disease processes, such as hypertension or diabetes.
Drug Sources
Historically, drugs came from plants, animals, and minerals. Now, most drugs are synthetic compounds manufactured in laboratories. Chemists, for example, often create useful new drugs by altering the chemical structure of existing drugs. Such techniques and other technologic advances have enabled the production of new drugs as well as synthetic versions of many drugs originally derived from plants and animals. Synthetic drugs are more standardized in their chemical characteristics, more consistent in their effects, and less likely to produce allergic reactions. Semisynthetic drugs (e.g., many antibiotics) are naturally occurring substances that have been chemically modified.
Biotechnology is also an important source of drugs. This process may involve manipulating deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) and recombining genes into hybrid molecules that can be inserted into living organisms (Escherichia coli bacteria are often used), which can be repeatedly reproduced. Each hybrid molecule produces a genetically identical molecule, called a clone. Cloning makes it possible to identify the DNA sequence in a gene and to produce the protein product encoded by a gene, such as insulin. Cloning also allows production of adequate amounts of the drug for therapeutic or research purposes. Biotechnology drugs constitute an increasing percentage of drugs now undergoing development, and this trend is expected to continue into the foreseeable future.
Drug Classifications and Prototypes
Drugs are classified according to their effects on particular body systems, their therapeutic uses, and their chemical characteristics. For example, morphine can be classified as a central nervous system depressant and a narcotic or opioid analgesic. The names of therapeutic classifications usually reflect the conditions for which the drugs are used (e.g., antidepressants, antihypertensives). However, the names of many drug groups reflect their chemical characteristics rather than their therapeutic uses (e.g., adrenergics, benzodiazepines). Many drugs fit into multiple groups because they have wide-ranging effects on the human body.
An individual drug that represents groups of drugs is called a prototype. The prototype, often the first drug of a particular drug class to be developed, is usually the standard with which newer drugs in the class are compared. For example, morphine is the prototype of the opioid analgesics and penicillin is the prototype of the beta-lactam antibacterial drugs.
Drug classifications and prototypes are quite settled, and most new drugs can be assigned to a group and compared with an established prototype. However, some groups lack a universally accepted prototype, and some prototypes are replaced over time by newer, more commonly used drugs. In this text, information about the prototype is provided for each drug class.
Drug Names
Individual drugs may have several different names, but the two that are most commonly used are the generic (official) name and the brand (trade) name. The generic name (e.g., amoxicillin) is related to the chemical or official name and is independent of the manufacturer. The generic name often indicates the drug group (e.g., drugs with generic names ending in “cillin” are penicillins). In the United States, the United States Adopted Names Council assigns the generic name. The brand (trade) name is designated and patented by the manufacturer. For example, amoxicillin is manufactured by several pharmaceutical companies, some of which assign a specific trade name (e.g., Amoxil, Trimox) and several of which use only the generic name. In drug literature, trade names are capitalized and generic names are presented in lowercase unless in a list or at the beginning of a sentence. Drugs may be prescribed and dispensed by generic or trade name. Generic equivalents are available for the majority of drugs and can be substituted for trade-named drugs unless the prescriber requests the trade-named medication by writing “do not substitute” on the prescription. Generic drugs are required to be therapeutically equivalent and are less expensive than trade name drugs.
NCLEX Success
1. The nurse is caring for a woman who has strong beliefs about not putting anything unnatural into her body. It is most accurate to say that most modern medications are
A. natural products derived from plants
B. natural products derived from minerals
C. synthetic products manufactured in laboratories
D. synthetic modifications of natural products
2. The nurse is taking care of a man who is confused about the different medications he is prescribed. He notes that some of the drug names have changed over the course of time he has been taking them. When counseling him, it is most important to keep the following statement in mind:
A. A drug can belong to only one group or classification.
B. A prototype drug is the standard by which similar drugs are compared.
C. Drug groups and prototypes change frequently, and knowledge about a prototype cannot guide knowledge about other drugs in the same class.
D. The generic name of a drug changes among manufacturers.
Drug Marketing
A patent protects a new drug for several years, during which time only the pharmaceutical manufacturer that developed it can market it. The company views this protection as a return on its investment in developing the drug, which might have required years of work and millions of dollars, and as an incentive to develop other drugs. Other pharmaceutical companies cannot manufacture and market the drug until the patent expires. However, for new drugs that are popular and widely used, other companies often produce similar drugs, with different generic and trade names.
Pharmacoeconomics
Pharmacoeconomics involves the costs of drug therapy, including costs of purchasing, dispensing (i.e., salaries of pharmacists, pharmacy technicians), storage, administration (i.e., salaries of nurses, costs of supplies), and laboratory and other tests used to monitor patient responses, as well as losses due to expiration. Length of illness or hospitalization is also a consideration. The goal of most pharmacoeconomic research is to identify drug therapy regimens that provide the desired benefits at the lowest cost.
Access to Drugs
Prescription and Nonprescription Drugs
Legally, American consumers have two ways to access therapeutic drugs. They can obtain them as prescription drugs, which require a written order. A licensed health care provider such as a physician, dentist, or nurse practitioner writes the prescription. Alternatively, they can purchase over-the-counter (OTC) drugs, which do not require a prescription. Various laws regulate these routes. Acquiring and using prescription drugs for nontherapeutic purposes by people who are not authorized to have the drugs or for whom they are not prescribed is illegal.
American Drug Laws and Standards
Current drug laws and standards have evolved over many years. Their main goal is to protect the public by ensuring that drugs marketed for therapeutic purposes are safe and effective. Table 1.1 further describes and summarizes the main provisions.

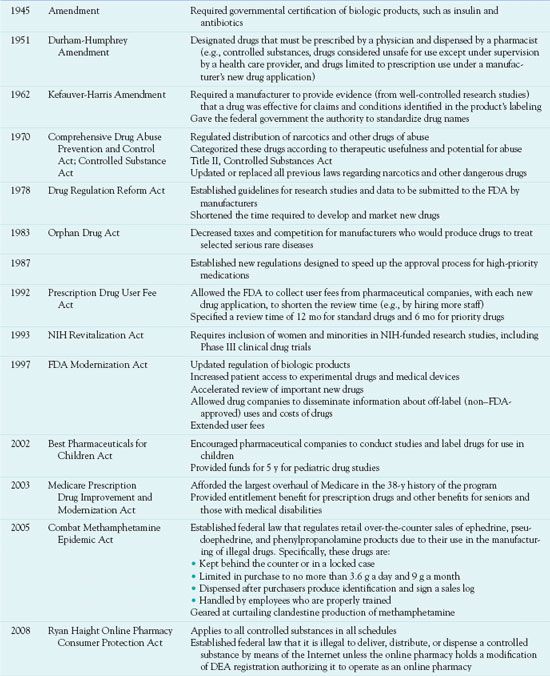
DEA, Drug Enforcement Administration; FDA, U.S. Food and Drug Administration; NIH, National Institutes of Health.
The Food, Drug, and Cosmetic Act of 1938 and its amendments regulate the manufacture, distribution, advertising, and labeling of drugs. The law also requires that official drugs (i.e., those listed in the United States Pharmacopeia and designated USP) must meet standards of purity and strength as determined by chemical analysis or by animal response to specified doses (bioassay). The Durham-Humphrey Amendment designates drugs that must be prescribed by a licensed physician or nurse practitioner and dispensed by a pharmacist. The U.S. Food and Drug Administration (FDA) is charged with enforcing the law. In addition, the Public Health Service regulates vaccines and other biologic products, and the Federal Trade Commission can suppress misleading advertisements of nonprescription drugs.
The Comprehensive Drug Abuse Prevention and Control Act was passed in 1970. Title II of this law, called the Controlled Substances Act, regulates the manufacture and distribution of narcotics, stimulants, depressants, hallucinogens, and anabolic steroids and requires the pharmaceutical industry to maintain physical security and strict recordkeeping for these drugs and substances. These drugs are categorized according to therapeutic usefulness and potential for abuse (Box 1.1) and are labeled as controlled substances (e.g., morphine is a C-II or Schedule II drug).
BOX 1.1 Categories of Controlled Substances
Schedule I
Drugs that have no accepted medical use, lack of accepted safety, and have high abuse potentials: heroin, lysergic acid diethylamide (LSD), marijuana, methaqualone (Quaalude), 3,4-methylenedioxy-methamphetamine (MDMA or ecstasy), mescaline, peyote, tetrahydrocannabinol.
Schedule II
Drugs that are used medically and have high abuse potentials: opioid analgesics (e.g., codeine, hydromorphone, methadone, meperidine, morphine, oxycodone), central nervous system (CNS) stimulants (e.g., cocaine, methamphetamine, methylphenidate), and barbiturate sedative-hypnotics (amobarbital, pentobarbital, secobarbital).
Schedule III
Drugs with less potential for abuse than those in Schedules I and II, but abuse may lead to psychological or physical dependence: androgens and anabolic steroids, some depressants (e.g., ketamine, pentobarbital, zolazepam), some CNS stimulants (e.g., benzphetamine, chlorphentermine), and mixtures containing small amounts of controlled substances (e.g., codeine, barbiturates not listed in other schedules). These drugs and substances have an accepted medical use in the United States.
Schedule IV
Drugs with an accepted medical use in the United States but with some potential for abuse: benzodiazepines (e.g., diazepam, lorazepam, temazepam), other sedative-hypnotics (e.g., phenobarbital, chloral hydrate), and some prescription appetite suppressants (e.g., mazindol, phentermine).
Schedule V
Products containing moderate amounts of controlled substances. They may be dispensed by the pharmacist without a physician’s prescription but with some restrictions regarding amount, recordkeeping, and other safeguards. Included are cough suppressants containing small amounts of codeine and antidiarrheal drugs, such as diphenoxylate and atropine (Lomotil).
The Drug Enforcement Administration (DEA) enforces the Controlled Substances Act. Individual people and companies legally empowered to handle controlled substances must be registered with the DEA, keep accurate records of all transactions, and provide for secure storage. The DEA assigns prescribers a number, which they must include on all prescriptions they write for a controlled substance. Prescriptions for Schedule II drugs cannot be refilled; a new prescription is required. Nurses are responsible for storing controlled substances in locked containers, administering them only to people for whom they are prescribed, recording each dose given on agency narcotic sheets and on the patient’s medication administration record, maintaining an accurate inventory, and reporting discrepancies to the proper authorities.
In addition to federal laws, state laws also regulate the sale and distribution of controlled drugs. These laws may be more stringent than federal laws; if so, the stricter laws usually apply.
Drug Approval Processes: Food and Drug Administration
The FDA is responsible for ensuring that new drugs are safe and effective before approving the drugs and allowing them to be marketed. The FDA reviews research studies (usually conducted or sponsored by pharmaceutical companies) about proposed new drugs; the organization does not test the drugs.
Testing Procedure
Since 1962, newly developed drugs undergo extensive testing before being marketed for general use. Initially, drug testing occurs in animals, and the FDA reviews the test results. Next, researchers perform clinical trials in humans, usually with a randomized, controlled experimental design that involves selection of subjects according to established criteria, random assignment of subjects to experimental groups, and administration of the test drug to one group and a control substance to another group.
Testing proceeds through several phases if there is continuing evidence of drug safety and effectiveness. In Phase I, a few doses are given to a certain number of healthy volunteers to determine safe dosages, routes of administration, absorption, metabolism, excretion, and toxicity. In Phase II, a few doses are given to a certain number of subjects with the disease or symptom for which the drug is being studied, and responses are compared with those of healthy subjects. In Phase III, the drug is given to a larger and more representative group of subjects. In double-blind, placebo-controlled designs, half of the subjects receive the new drug and half receive a placebo (an inactive substance similar in appearance to the actual drug), with neither subjects nor researchers knowing who receives which formulation. In crossover studies, subjects serve as their own control; each subject receives the experimental drug during half of the study and a placebo during the other half. Other research methods include control studies in which some patients receive a known drug rather than a placebo; in subject matching, patients are paired with others of similar characteristics. Phase III studies help determine whether the potential benefits of the drug outweigh the risks. Testing may be stopped during any of the early phases if inadequate effectiveness or excessive toxicity becomes evident. In Phase IV, the FDA evaluates the data from the first three phases for drug safety and effectiveness, allows the drug to be marketed for general use, and requires manufacturers to continue monitoring the drug’s effects.
Historically, drug research involved mainly young, white males. In 1993, Congress passed the National Institutes of Health (NIH) Revitalization Act, which formalized a policy of the NIH that women and minorities be included in human subject research studies funded by the NIH and that women and minorities be included in clinical drug trials. Now, major drug trials must recruit female subjects and include outcome data on women. In addition, all newly developed drugs must include gender-related effectiveness and safety information in the initial FDA application. Knowledge about the drug effects in women has increased but is still relatively limited because many commonly used drugs were developed before enactment of these regulations.
Subsequent withdrawal of some approved and marketed drugs (e.g., Vioxx) may occur, usually because of serious adverse effects that become evident only when the drugs are used in a large, diverse population. In addition, in recent years, the FDA has issued warnings about several drugs that can cause serious adverse effects (e.g., antidepressants, nonsteroidal anti-inflammatory drugs [NSAIDs] such as aspirin and ibuprofen, and oseltamivir [Tamiflu], an antiflu drug). As a result, the FDA has received criticism for approving these and other drugs and allowing them to be marketed in the first place. Some authorities are urging major reforms in the drug approval process, the methods of reporting adverse drug effects, and postmarketing surveillance procedures.
Drug Approval
The FDA approves many new drugs annually. New drugs are categorized according to their review priority and therapeutic potential. A status of “1P” indicates a new drug reviewed on a priority (accelerated) basis and with some therapeutic advantages over drugs already available. A status of “1S” indicates standard review and drugs with few, if any, therapeutic advantages (i.e., the new drug is similar to one or more older drugs currently on the market). Most new drugs are “1S” prescription drugs.
The FDA also approves drugs for OTC availability, including the transfer of drugs from prescription to OTC status, and may require additional clinical trials to determine the safety and effectiveness of OTC use. For prescription drugs taken orally, transfer to OTC status may mean different indications for use and lower doses. FDA approval of a drug for OTC availability involves evaluation of evidence that the consumer can use the drug safely, using information on the product label, and shifts primary responsibility for safe and effective drug therapy from health care professionals to consumers. With prescription drugs, a health care professional diagnoses the condition, often with the help of laboratory and other diagnostic tests, and determines a need for the drug. With OTC drugs, the patient must make these decisions, with or without consultation with a health care provider.
Having drugs available over the counter has potential advantages and disadvantages for consumers. Advantages include greater autonomy, faster and more convenient access to effective treatment, possibly earlier resumption of usual activities of daily living, fewer visits to a health care provider, and possibly increased efforts by consumers to learn about their symptoms/conditions and recommended treatments. Disadvantages include inaccurate self-diagnoses and potential risks of choosing a wrong or contraindicated drug, delayed treatment by a health care professional, and development of adverse drug reactions and interactions. When a drug is switched from prescription to OTC status, sales and profits of pharmaceutical companies increase and costs of insurance companies decrease. Costs to consumers increase because health insurance policies do not cover OTC drugs.
Clinical Application 1-1
Ms. Clark analyzes drug safety, including the national organizations charged with ensuring it.
 What is the role of the U.S. Food and Drug Administration (FDA) in the drug approval process?
What is the role of the U.S. Food and Drug Administration (FDA) in the drug approval process?
 What is the role of the Drug Enforcement Administration (DEA) and the nurse with regard to controlled substances?
What is the role of the Drug Enforcement Administration (DEA) and the nurse with regard to controlled substances?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


