Cheryl J. Dumont
Infusion Therapy*
Learning Outcomes
Safe and Effective Care Environment
Health Promotion and Maintenance
Physiological Integrity
6 Check the accuracy of prescriptions for IV fluids and drug therapy.
7 Identify the appropriate veins for peripheral IV catheter insertion.
8 Differentiate types of vascular access devices (VADs) used for peripheral and central IV therapy.
9 Use best practice for inserting peripheral vascular access devices (VADs).
11 Prioritize nursing interventions for maintaining an infusion system.
12 Assess, prevent, and manage systemic complications related to infusion therapy and VADs.

http://evolve.elsevier.com/Iggy/
Answer Key for NCLEX Examination Challenges and Decision-Making Challenges
Audio Glossary
Concept Map Creator
Key Points
Review Questions for the NCLEX® Examination
Infusion therapy is the delivery of medications in solution and fluids by parenteral (piercing of skin or mucous membranes) route through a wide variety of catheter types and locations using multiple procedures. IV therapy is the most common route for infusion therapy. It delivers solutions directly into the veins of the vascular system. This chapter focuses on access for and administration of all types of infusion therapy.
Overview
Infusion therapy is delivered in all health care settings, including hospitals, home care, ambulatory clinics, physicians’ offices, and long-term care facilities. The most common reasons for using infusion therapy are to:
• Maintain fluid balance or correct fluid imbalance
• Maintain electrolyte or acid-base balance or correct electrolyte or acid-base imbalance
IV therapy is the most common invasive therapy administered to hospitalized patients. Advances in medicine and technology have made it possible for people with chronic diseases such as diabetes mellitus, chronic kidney disease, and malabsorption syndromes to live long and productive lives. These patients are often dependent on long-term infusion therapy of some kind. They often have very poor vascular integrity and, therefore, accessing their peripheral veins takes a high level of skill.
Having a specialized team of infusion nurses to initiate and maintain infusion therapy is recommended by the Centers for Disease Control and Prevention (CDC) to reduce complications of infusion therapy (O’Grady et al., 2011). These teams have demonstrated value in cost savings, patient satisfaction, and patient outcomes.
Infusion nurses may perform any or all of the following activities:
• Develop evidence-based policies and procedures
• Educate staff, patients, and families
• Consult on product selection and purchasing decisions
The registered nurse (RN) generalist is taught to insert peripheral IVs, and most institutions have a process of credentialing this skill. Depending on the state’s nurse practice act, licensed practical/vocational nurses (LPNs/LVNs) and technicians can be trained and credentialed to perform the skill of peripheral IV insertion and assist with infusions, but the RN is ultimately accountable for all aspects of infusion therapy and delegation of associated tasks (Infusion Nurses Society [INS], 2006).
The Infusion Nurses Society (INS) publishes guidelines and standards of practice for policy and procedure development in all health care settings. These standards establish the criteria for all nurses delivering infusion therapy. The Infusion Nurses Certification Corporation (INCC) offers a written certifying examination. Nurses who successfully complete this examination have mastered an advanced body of knowledge in this specialty and may use the initials CRNI, which stand for certified registered nurse infusion.
Types of Infusion Therapy Fluids
Many types of parenteral fluids are used for infusion therapy. These fluids include:
Intravenous Solutions
More than 200 IV fluids (solutions) are available that meet the requirements established by the United States Pharmacopeia (USP). Each solution is classified by its tonicity (concentration) and pH. Tonicity is typically categorized by comparison with normal blood plasma as osmolarity (mOsm/L). As discussed in Chapter 13, normal serum osmolarity for adults is between 270 and 300 mOsm/L. Parenteral solutions within that normal range are isotonic; those fluids greater than 300 mOsm/L are hypertonic; and those fluids less than 270 mOsm/L are hypotonic. Table 13-4 in Chapter 13 lists common IV solutions according to their tonicity.
When an isotonic infusate (solution that is infused into the body) is used, water does not move into or out of the body’s cells. Therefore patients receiving isotonic solutions are at risk for fluid overload, especially older adults. This complication is discussed in Chapter 13. Hypertonic fluids are used to correct fluid, electrolyte, and acid-base imbalances by moving water out of the body’s cells and into the bloodstream. Parenteral nutrition solutions are also hypertonic (see Chapter 63). Instead of moving water out of cells, hypotonic infusates move water into cells to expand them. Patients receiving either hypertonic or hypotonic fluids are at risk for phlebitis and infiltration. Phlebitis is the inflammation of a vein caused by mechanical, chemical, or bacterial irritation. Infiltration occurs when IV solution leaks into the tissues around the vein.
The pH of IV solutions measures the acidity or alkalinity and usually ranges from 3.5 to 6.2. Extremes of both osmolarity and pH can cause vein damage leading to phlebitis and thrombosis (blood clot in the vein). Thus fluids and medications with a pH value less than 5 and more than 9 and with an osmolarity more than 600 mOsm/L are best infused in the central circulation where greater blood flow provides adequate hemodilution (Perucca, 2010). For example, total parenteral nutrition (TPN) solutions have an osmolarity greater than 1400 mOsm/L. TPN should not be infused in peripheral circulation because it can damage blood cells and the endothelial lining of the veins.
Blood and Blood Components
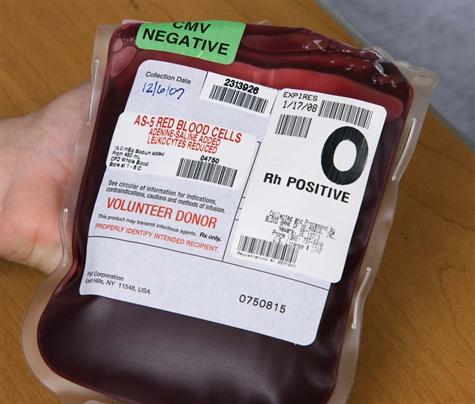
Blood transfusion is given by using packed red blood cells, created by removing a large portion of the plasma from whole blood. Other available blood components include platelets, fresh frozen plasma, albumin, and several specific clotting factors. Each component has detailed requirements for blood-type compatibility and infusion techniques. For patient safety, The Joint Commission’s 2010 National Patient Safety Goals (NPSGs) require agencies to ensure that blood components are properly ordered, handled and dispensed, and administered and that patients are appropriately monitored. Positive patient identification using two patient identifiers and requiring two qualified health care professionals is essential before any blood or blood component is administered. Most organizations use the International Society of Blood Transfusion (ISBT) universal bar-coding system to ensure the right blood for the right patient (Fig. 15-1). The ISBT system includes four components that must be present on the blood label both in bar code and in eye-readable format. These four components are (1) a unique facility identifier, (2) the lot number relating to the donor, (3) the product code, and (4) the ABO group and Rh type of the donor.
An acute hemolytic transfusion reaction caused by an incompatible blood transfusion is a “sentinel event” requiring an intense analysis of the contributing factors and corrective action. Established policies and procedures must be rigidly followed to ensure a safe transfusion and reduce the risk for complications. (See Chapter 42 for a complete discussion of blood transfusion administration.)
Drug Therapy
IV drugs provide a rapid therapeutic effect but can lead to immediate serious reactions, called adverse drug events (ADEs). Hundreds of drugs are available for infusion by a variety of techniques. As with all drug administration, nurses must be knowledgeable about drug indications, proper dosage, contraindications, and precautions. IV administration also requires knowledge of appropriate dilution, rate of infusion, pH and osmolarity, compatibility with other IV medications, appropriate infusion site (peripheral versus central circulation), potential for vesicant/irritant effects, and specific aspects of patient monitoring because of its immediate effect. Regardless of familiarity with the drug, never assume that IV administration is the same as giving that drug by other routes. New information is continuously being published, and new drugs are rapidly being introduced.
Medication safety is extremely important in all health care settings today. The Joint Commission publishes new and updated National Patient Safety Goals (NPSGs) every year. One major goal is improving the safety of high-alert drugs. An example of these drugs is concentrated electrolyte solutions (e.g., potassium chloride), which require restricted access, prominent warnings about the concentration, and storage in a secured location.
Procedures must be established to prevent errors resulting from look-alike, sound-alike drugs such as Celebrex IV (celecoxib) and Cerebyx (fosphenytoin). Other strategies to reduce errors include limiting available concentrations of drugs and dispensing all drugs, including catheter flush solutions, in single-dose containers. Smart pumps, with drug libraries (see Infusion Systems section), in combination with computer physician order entry (CPOE) and barcode medication administration (BCMA) systems, use recent technology to help reduce adverse drug events (ADEs). Electronic medication administration records (MARs) and multiple checks by pharmacists, as required by The Joint Commission’s NPSGs, also help reduce errors.
Prescribing Infusion Therapy
A prescription for infusion therapy written by a physician or other authorized health care provider is necessary before IV therapy begins. To be complete, the prescription for infusion fluids should include:
A drug prescription should include:
• Drug name, preferably by generic name
• Purpose (required in some health care agencies, especially nursing homes)
Some continuously infused drugs, such as those for pain management, are prescribed as milligrams per hour. The type and volume of dilution for infusion medications may be included in the prescription or calculated by the infusion pharmacist.
Vascular Access Devices
An infusion catheter, also known as a vascular access device (VAD), is a plastic tube placed in a blood vessel to deliver fluids and medications. The specific type and purpose of the therapy determine whether the infusion can be given safely through peripheral veins or if the large central veins of the chest are needed. Advances in catheter materials and insertion techniques have radically expanded the types of VADs currently used. This discussion includes the description of each type of catheter used for peripheral and central IV therapy. Seven major types are described:
• Peripherally inserted central catheters (PICC)
• Nontunneled percutaneous central venous catheters (CVCs)
Assess the patient’s needs for vascular access, and choose the device that has the best chance of infusing the prescribed therapy for the required length of time. Depending on the patient and type of VAD to be inserted, a topical anesthetic agent or intradermal lidocaine HCl 1% may be helpful to decrease patient discomfort. Obtain a health care provider’s order and check for patient allergies before administering any anesthetic.
Peripheral Intravenous Therapy
Short infusion catheters are the most commonly used vascular access devices (VADs) for peripheral IV therapy. They are usually placed in the veins of the arm. Another catheter used for peripheral IV therapy is a midline catheter.
Short Peripheral Catheters
Short peripheral catheters are composed of a plastic cannula built around a sharp stylet extending slightly beyond the cannula (Fig. 15-2). The stylet (sharp) allows for the venipuncture, and the cannula is advanced into the vein. Once the cannula is advanced into the vein, the stylet is withdrawn. These catheters are designed with a safety mechanism to cover the sharp end of the stylet after it is removed from the patient. These stylets are hollow-bore, blood-filled needles that carry a high risk for exposure to bloodborne pathogens if needle stick injury occurs. A federal law enacted in 2000 amended the Bloodborne Pathogen Standards from the Occupational Safety and Health Administration (OSHA) requiring the use of catheters with an engineered safety mechanism to prevent needle sticks.
Insertion and Placement Methods
Short peripheral catheters are usually inserted into superficial veins of the dorsal surface of the hand and the forearm using sterile technique. In emergent situations, these catheters can be used also in the external jugular vein of the neck. Avoid the use of veins in the lower extremities of adults, if possible, because of an increased risk for deep vein thrombosis and infiltration.
Short catheters range in length from 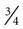 inch to
inch to 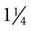 inch with gauge sizes from 26 gauge (the smallest) to 14 gauge (large bore). Choose the smallest gauge catheter capable of delivering the prescribed therapy. Current design allows for a thin-wall construction, providing a larger lumen (opening) without increasing the outer diameter. This design improves the fluid flow through the catheter while using a smaller gauge and thereby decreases the possibility of vein irritation from a large catheter. For example, a thin-walled 24-gauge Insyte (BD Medical) catheter has about the same flow-rate ability as a 22-gauge non–thin-walled Angiocath (BD Medical). Larger gauge sizes allow for faster flow rates but also cause phlebitis more often. Table 15-1 lists each gauge size and its common uses.
inch with gauge sizes from 26 gauge (the smallest) to 14 gauge (large bore). Choose the smallest gauge catheter capable of delivering the prescribed therapy. Current design allows for a thin-wall construction, providing a larger lumen (opening) without increasing the outer diameter. This design improves the fluid flow through the catheter while using a smaller gauge and thereby decreases the possibility of vein irritation from a large catheter. For example, a thin-walled 24-gauge Insyte (BD Medical) catheter has about the same flow-rate ability as a 22-gauge non–thin-walled Angiocath (BD Medical). Larger gauge sizes allow for faster flow rates but also cause phlebitis more often. Table 15-1 lists each gauge size and its common uses.
TABLE 15-1
CHOOSING THE GAUGE SIZE FOR PERIPHERAL CATHETERS
| CATHETER GAUGE | INDICATIONS | APPROXIMATE FLOW RATES |
| 24-26 gauge Smallest, shortest ( 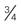 -inch length) -inch length) | Not ideal for viscous infusions Expect blood transfusion to take longer Preferred for infants and small children | 24 mL/min (1440 mL/hr) |
| 22 gauge | Adequate for most therapies, blood can infuse without damage | 38 mL/min (2280 mL/hr) |
20 gauge (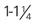 -inch length) -inch length) | Adequate for all therapies Most anesthesiologists prefer not to use a smaller size than this for surgery cases | 65 mL/min (3900 mL/hr) |
| 18 gauge | Preferred size for surgery Vein needs to be large enough to accommodate the catheter | 110 mL/min (6600 mL/hr) |
| 14-16 gauge | For trauma and surgical patients requiring rapid fluid resuscitation Needs to be in a vein that can accommodate it |
Short peripheral catheters are allowed to dwell (stay in) for 72 to 96 hours but then require removal and insertion at another venous site. If the patient’s therapy is expected to be longer than 6 days, a midline catheter or PICC should be chosen (O’Grady et al., 2011). When selecting the site for insertion of a peripheral catheter, consider the patient’s age, history, and diagnosis; the type and duration of the prescribed therapy; and, whenever possible, the patient’s preference. Chart 15-1 lists the major criteria for the placement of peripheral VADs.
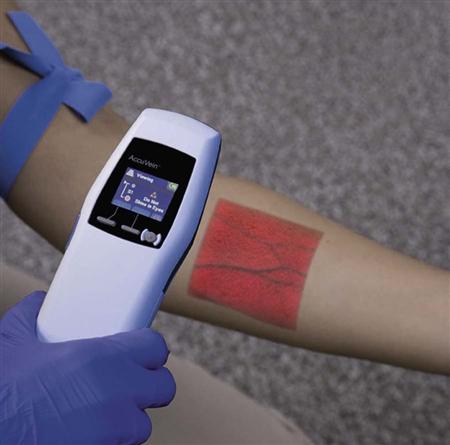
Though not yet widely used in most clinical settings, vein transilluminators and ultrasound devices are now available as tools to assist in IV line placement. Several different types of portable vein transilluminators are available, such as VeinViewer, Veinlite LED, and AccuVein (Fig. 15-3). Although they may have different mechanisms of action, some using infrared light and some using laser, these devices penetrate only up to approximately 10 mm and so are limited to finding superficial veins.
Ultrasound-guided peripheral IV insertion can allow insertion into deeper veins. Although this technology is very valuable, it may increase the risks for complications. Arteries and nerves lie parallel to deep veins, and special training is needed to learn to identify these structures and avoid damaging them. In addition, when deeper veins are accessed, infiltration may go undetected until a significant amount of fluid has collected in the tissues. This complication can be particularly devastating if the solution is an irritant or vesicant. If these devices are not used, nurses must rely on sight and touch to insert an IV catheter.
For patients who need IV access but are at risk for fluid overload or do not need extra IV fluids, the peripheral vascular access device (VAD) can be converted into an intermittent IV lock, also called a saline lock. This device allows administration of specific drugs given IV push (e.g., furosemide [Lasix, Furoside ![]() ]) or on an intermittent basis using a medication administration set. IV antibiotics are frequently given this way. In some cases, the saline lock is placed in case there is a need for emergency drug administration via IV push. The intermittent device is flushed with saline before and after drug administration to ensure patency and prevent occlusion with a blood clot.
]) or on an intermittent basis using a medication administration set. IV antibiotics are frequently given this way. In some cases, the saline lock is placed in case there is a need for emergency drug administration via IV push. The intermittent device is flushed with saline before and after drug administration to ensure patency and prevent occlusion with a blood clot.
Site Selection and Skin Preparation
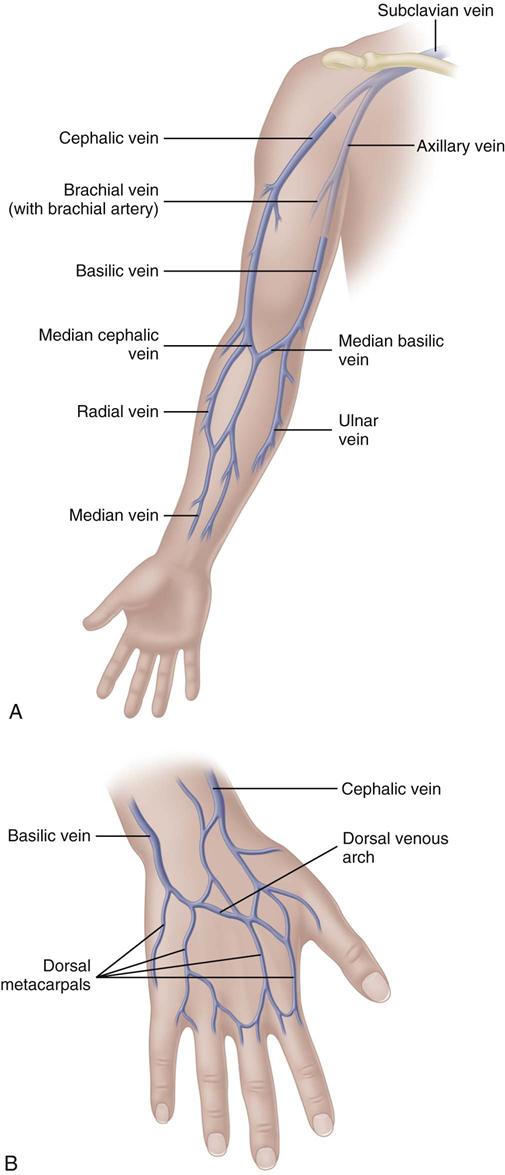
The most appropriate veins for peripheral catheter placement include the dorsal venous network, basilic, cephalic, and median veins, as well as their branches (Fig. 15-4). However, cannulation of veins on the hand is not appropriate for older patients with a loss of skin turgor and poor vein condition and for active patients receiving infusion therapy in an ambulatory clinic or home care. Use of veins on the dorsal surface of the hands should be reserved for short-term infusion of non-vesicant and non-irritant solutions.
Mastectomy, axillary lymph node dissection, lymphedema, paralysis of the upper extremity, and the presence of dialysis grafts or fistulas alter the normal pattern of blood flow through the arm. Using veins in the extremity affected by these conditions requires a physician’s request. Short peripheral catheters are not recommended for obtaining routine blood samples.
Winged needles (“butterfly needles”) are easy to insert but are associated with a high frequency of infiltration. They are most commonly used for injection of single-dose drugs or for drawing blood samples. Like a short peripheral catheter, winged needles should also have an engineered safety mechanism to house the needle when removed.
Aseptic skin preparation and technique before IV insertion are crucial. Catheter-related bloodstream infections (CR-BSI) can occur from a peripheral IV site. The CDC recommendations include:
Midline Catheters
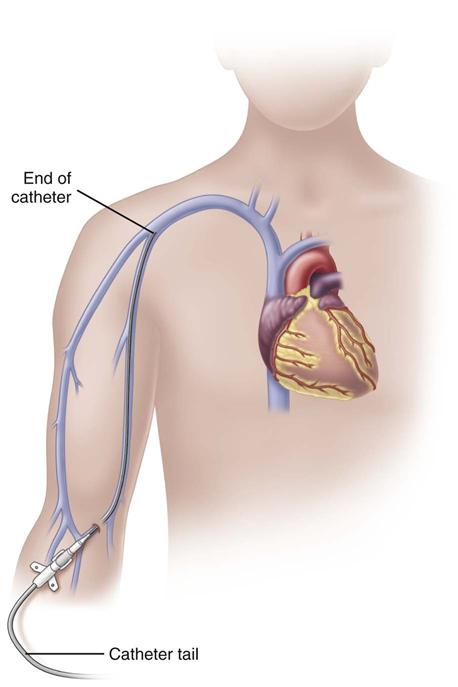
Midline catheters can be anywhere from 3 to 8 inches long, 3 to 5 Fr, and double or single lumen. They are inserted through the veins of the upper arm. The median antecubital vein is used most often if insertion is done without the aid of ultrasound guidance. With ultrasound guidance, deeper veins can be accessed and the insertion site can be further above the antecubital fossa. The basilic vein is preferred over the cephalic vein because of its larger diameter and straighter path. It also allows greater hemodilution of the fluids and medications being infused. The catheter tip is located in the upper arm with the tip residing no further into the venous network than the axillary vein (Fig. 15-5). These catheters are used for therapies lasting from 1 to 4 weeks; however, there are no recommendations for the optimal dwell time. Because of the extended dwell time, strict sterile technique is used for insertion and dressing changes for a midline catheter (Rosenthal, 2008). Additional education and skill assessment are required for the nurse to be qualified to insert midline catheters.
A midline catheter can be used when skin integrity or limited peripheral veins make it difficult to maintain a short peripheral catheter. Patients who have received anticoagulation and/or steroid therapy can also benefit from avoidance of repeated IV sticks and the subsequent excessive bruising and hematoma formation.
• Longer than 6 days and up to 4 weeks of therapy such as:
• Heparin infusions for deep vein thrombosis
• Bronchodilators, such as aminophylline
• Steroids
Midline catheters are considered to dwell in the peripheral circulation; the recommendations for infusates are the same as for short peripheral IVs. Fluids and medications infused through a midline catheter should have a pH between 5 and 9 and a final osmolarity of less than 600 mOsm/L (Perucca, 2010; Rosenthal, 2008). The pH and osmolarity outside these parameters increase the risk for complications like phlebitis and thrombosis. Midline catheters should not be used for infusion of vesicant medications—drugs that cause severe tissue damage if they escape into the subcutaneous tissue (extravasation). At a midline tip location, larger amounts of the drug can extravasate before the problem is detected.
All parenteral nutrition formulas, including those with low concentrations of dextrose, and solutions that have an osmolarity greater than 600 mOsm/L should not be infused through a midline catheter. Do not draw blood from these catheters routinely. Midline catheters should not be placed in extremities affected by mastectomy with lymphedema, paralysis, or dialysis grafts and fistulas. When using a double-lumen midline catheter, do not administer incompatible drugs simultaneously administered through both lumens because the blood flow rate in the axillary vein is not high enough to ensure adequate hemodilution and prevention of drug interaction in the vein.
Some midline catheters are designed with pressure-sensitive valves located near the internal catheter tip (e.g., Groshong, made by Bard Access Systems) or in the external catheter hub (e.g., PASV, made by Boston Scientific). These valves open when pressure is applied, allowing for infusion and aspiration. However, when no pressure is applied, they are closed to prevent blood refluxing (backing) into the lumen or air entering the bloodstream. The manufacturers of both catheters state to use only a saline flush for lines with pressure-sensitive valves.
Central Intravenous Therapy
In central IV therapy, the vascular access device (VAD) is placed in the central circulation, specifically within the superior vena cava (SVC) near its junction with the right atrium. Blood flow in the SVC is approximately 2 L/min compared with about 200 mL/min in the axillary vein. All central vascular access devices require confirmation of tip location by chest radiograph before solutions are infused (Hadaway, 2010a). However, the use of newer ultrasound systems, such as the Sherlock PICC tip location system, can decrease the need for repeated x-rays by confirming approximate catheter position. The practitioner inserts a specially designed magnetic stylet through the catheter before insertion into the patient so that the system can detect its tip location. These devices have been endorsed by the CDC and Agency for Healthcare Research and Quality (Moreau, 2008a).
A number of types of central vascular access devices (CVADs) are available, depending on the purpose, duration, and insertion site availability. Several recent improvements in catheter materials allow antimicrobial and heparin coatings to reduce infection risk and improve the longevity of the catheter. Newer central catheters also allow for rapid injection rates required for contrast studies.
Peripherally Inserted Central Catheters
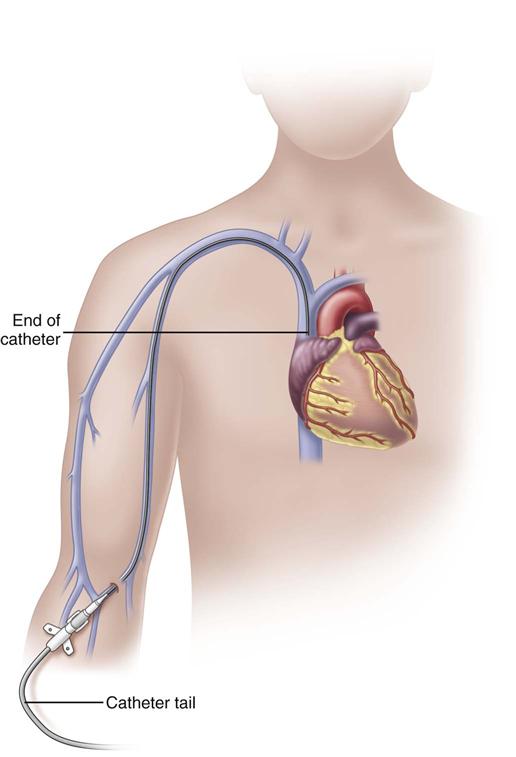
A peripherally inserted central catheter (PICC) is a long catheter inserted through a vein of the antecubital fossa (inner aspect of the bend of the arm) or the middle of the upper arm. Nurses who insert these CVADs require special training and certification.
In adults, the PICC length ranges from 18 to 29 inches (45-72 cm) with the tip residing in the superior vena cava (SVC) (Fig. 15-6). Placement of the catheter tip in veins distal to the SVC is avoided. This inappropriate tip location, often called a mid-clavicular catheter, is associated with much higher rates of thrombosis than when the tip is located in the SVC. Mid-clavicular tip locations are used only when anatomic or pathophysiologic changes prohibit placing the catheter into the SVC.
PICCs should be inserted early in the course of therapy before veins of the extremity have been damaged from multiple venipunctures and infusions. Insertion methods using guidewires and ultrasound systems greatly improve insertion success. The basilic vein is the preferred site for insertion; the cephalic vein can be used if necessary. Sterile technique is used for insertion to reduce the risk for catheter-related bloodstream infections (CR-BSIs). Before the catheter can be used for infusion, a chest x-ray indicating that the tip resides in the lower SVC is required when the catheter is not placed under fluoroscopy.
PICCs are available in single-, dual-, or triple-lumen configurations and are available with both the Groshong valve and the pressure-activated safety valve (PASV). PICCs are also available as “Power PICCs” and can be used for contrast injection at a maximum of 5 mL/sec and a maximum pressure of 300 psi. They can also be connected to transducers and used to monitor central venous pressure (Bard Access Systems, 2011).
The most common complications from PICCs include phlebitis, thrombophlebitis, and CR-BSIs. Thrombophlebitis can be very serious and threatens the integrity of the vein. CR-BSI has been noted to be less frequent in PICCs than in other central venous catheters (CVCs) because of the insertion site in the upper extremity. The cooler, drier skin of the upper arm has fewer types and numbers of microorganisms, leading to lower rates of infection. Accidental arterial puncture or excessive bleeding can occur on insertion and is controlled by direct pressure. Infiltration and extravasation are rare. Insertion complications such as pneumothorax associated with other CVCs do not occur with PICCs.
PICCs can accommodate infusion of all types of therapy because the tip resides in the SVC where the rapid blood flow quickly dilutes the fluids being infused. Therefore there are no limitations on the pH or osmolality of fluids that can be infused through a PICC. For example, patients requiring lengthy courses of antibiotics, chemotherapy agents, parenteral nutrition formulas, and vasopressor agents can benefit from this type of catheter. PICCs have been reported to dwell successfully for months or even years; however, the optimal dwell time is not known.
PICCs can be used for blood sampling; however, lumen sizes of 4 Fr or larger are recommended. Using lumens with small diameters may not yield a sample capable of producing the needed test results. Transfusion of blood through a PICC usually requires the use of an infusion pump. Packed red blood cells are cold and viscous. The length of the catheter adds resistance and may prevent the blood from infusing within the 4-hour limit.
Teach patients with a PICC to perform usual ADLs; however, they should avoid excessive physical activity. Muscle contractions in the arm from physical activity like heavy lifting can lead to catheter dislodgment and possible lumen occlusion. PICCs may be contraindicated in paraplegic patients who rely on their arms for mobility.
PICC insertion is commonly performed in the patient’s hospital room, an outpatient treatment facility, or the imaging department. Regardless of where they are inserted, the same precautions must be taken as with any other central line insertion using the catheter-related bloodstream infection (CR-BSI) prevention bundle. Major components of this prevention bundle include:
• Maximal barrier precautions upon insertion
• Chlorhexidine skin antisepsis
• Daily review of line necessity with prompt removal of unnecessary lines
Nontunneled Percutaneous Central Venous Catheters
Nontunneled percutaneous central venous catheters (CVCs) are inserted by a physician through the subclavian vein in the upper chest or the internal jugular veins in the neck using sterile technique. Occasionally the patient’s condition may require insertion of the CVC in a femoral vein, but the rate of infection is very high. If the femoral site must be used, it is removed as soon as possible.
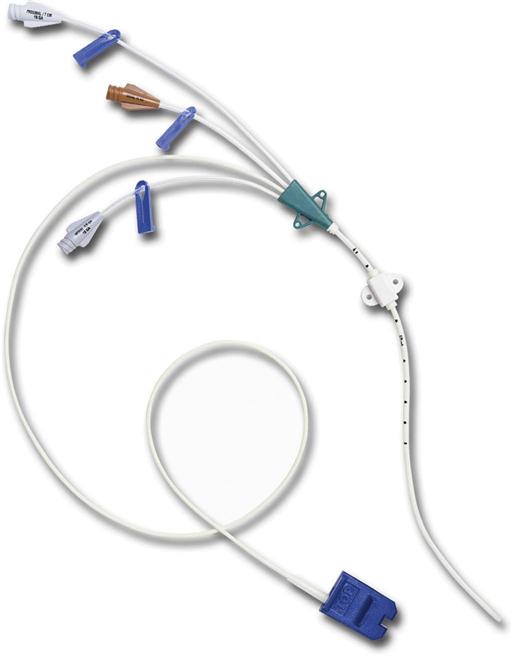
CVCs are usually 7 to 10 inches (18 to 25 cm) long and have one to as many as five lumens (Fig. 15-7). These catheters are also available with antimicrobial coatings such as chlorhexidine and silver sulfadiazine. The tip resides in the superior vena cava (SVC) and is confirmed by a chest x-ray. Nontunneled percutaneous CVCs are most commonly used for emergent or trauma situations, critical care, and surgery. There is no recommendation for optimal dwell time. However, these catheters are commonly used for short-term situations and are not the catheter of choice for home care or ambulatory clinic settings.
Insertion of these central catheters requires the patient to be placed in the Trendelenburg position, usually with a rolled towel between the shoulder blades. This position may be difficult or contraindicated for patients with respiratory conditions, spinal curvatures, and increased intracranial pressure, especially for older adults. Trauma, surgery, or radiation in the neck or chest prohibits the use of these devices as well. The presence of a tracheotomy increases the risk for cross-contamination of the insertion site. The warmer, moister skin of the neck and upper chest has more types and higher numbers of microorganisms, resulting in more CR-BSIs with this type of catheter.
Tunneled Central Venous Catheters
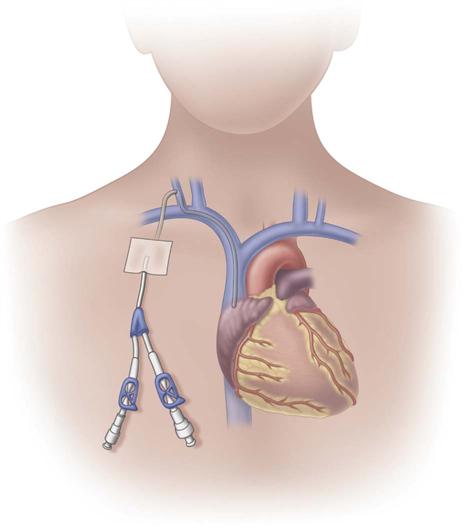
Tunneled central venous catheters are VADs that have a portion of the catheter lying in a subcutaneous tunnel, separating the points where the catheter enters the vein from where it exits the skin. This separation is intended to prevent the organisms on the skin from reaching the bloodstream (Fig. 15-8). The catheter has a cuff made of a rough material that is positioned inside the subcutaneous tunnel. These cuffs commonly contain antibiotics, which also reduce the risk for infection. The tissue granulates into the cuff, providing a mechanical barrier to microorganisms and anchoring the catheter in place.
The design of tunneled CVCs requires surgical techniques for insertion and removal. Single-, dual-, and triple-lumens are available. These catheters were originally named for the physicians who designed them, including Broviac, Hickman, and Leonard catheters.
Tunneled catheters are used primarily when the need for infusion therapy is frequent and long-term. Patients needing parenteral nutrition for months, years, or the remainder of their life commonly choose a tunneled catheter. Tunneled catheters are also chosen when several weeks or months of infusion therapy are needed and a PICC is not a good choice. For example, paraplegic patients needing 6 to 8 weeks of antibiotics are not good candidates for a PICC because of the excessive use of the upper extremities for mobility. Some oncology patients may prefer a tunneled catheter instead of an implanted port because they cannot tolerate the needle sticks required for accessing those devices.
Implanted Ports
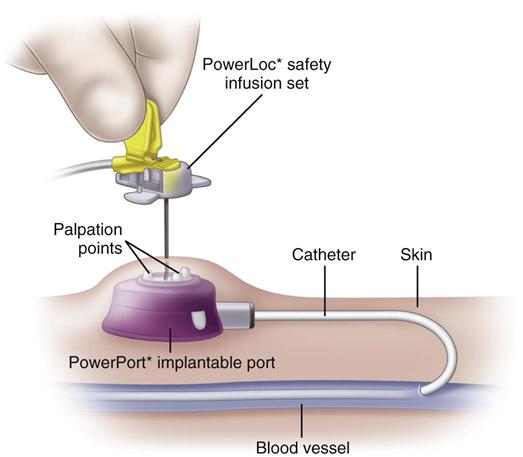
Implanted ports are very different from other central vascular access devices (CVADs). They consist of a portal body, a dense septum over a reservoir, and a catheter. They can be single- or double-lumen and come in various sizes. A subcutaneous pocket is surgically created to house the port body. The catheter is inserted into the vein and attached to the portal body. The septum is made of self-sealing silicone and is located in the center of the port body over the reservoir; the catheter extends from the side of the port body. The incision is closed, and no part of the catheter is visible externally; therefore this device has the least impact on body image (Fig. 15-9).
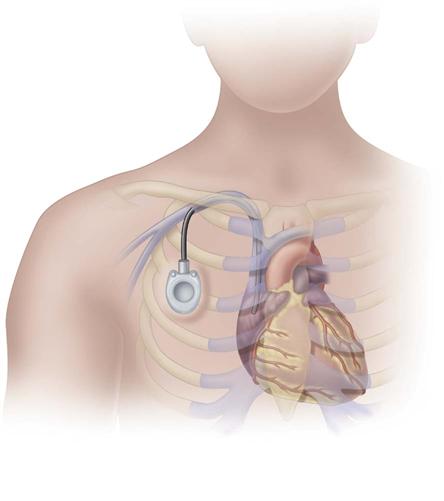
Some implanted ports are power-injectable and can be used for obtaining contrast-enhanced computed tomography (CECT). These devices can withstand 5 mL/sec at up to 300 psi pressure. The BARD PowerPort can be identified by palpation of three bumps on the top of the septum and a triangular-shaped port. Be careful not to press firmly on the bumps because it can be painful to the patient. Be sure to use a power-injection–rated noncoring needle with this type of port when it is used for this purpose. These needles come with labeling identifying that they are power-injection rated (Fig. 15-10).
Venous ports may be placed on the upper chest or the upper extremity. The venous catheter may enter either the subclavian or internal jugular vein. Although an implanted port is most commonly used in the venous system, the catheter may be placed in arteries, the epidural space, or the peritoneal cavity, with the port pocket located over a bony prominence.
Implanted ports are accessed by using a noncoring needle (a common brand name is Huber) that is specially designed with a deflected tip (Fig. 15-11). This design slices through the dense septum without coring out a small piece of it, thus preserving the integrity of the septum. Port bodies placed in the chest have a larger septum and usually tolerate about 2000 punctures. Port bodies placed in the upper extremity are smaller and are rated to tolerate about 750 punctures.
Before puncture, palpate the port to locate the septum. Carefully palpate to feel the shape and depth of the port body to ensure puncture of the septum, not the attached catheter. Some have attached extension sets and wings to stabilize the needle. One important feature is an engineered safety mechanism to contain the needle when it is removed from the septum. Because the dense septum holds tightly to the needle, there can be a rebound when it is pulled from the septum, often resulting in needle stick injury to the nurse.
Implanted ports need to be flushed after each use and at least once a month between courses of therapy. This procedure is done to prevent clot formation in the internal chamber of the port and is often referred to as “locking” or “de-accessing.” The INS recommendation for locking or de-accessing a port is 5 mL of 100 units heparin/mL. However, locking a central line to prevent it from clotting is controversial and under-researched (Gorski et al., 2010). When the port is not accessed, there is no external catheter requiring a dressing. Puncture of the skin over the port is required to gain access to the port body, causing pain for some patients. Topical anesthetic creams can be used to make the access procedure more tolerable.
Hemodialysis Catheters
Hemodialysis catheters have very large lumens to accommodate the hemodialysis procedure or a pheresis procedure that harvests specific blood cells. They may be tunneled for long-term needs or nontunneled for short-term needs. A hemodialysis catheter is critical to the management of renal failure and must function well. Catheter-related bloodstream infections (CR-BSIs) and vein thrombosis are common problems; therefore this catheter should not be used for administration of other fluids or drugs except in an emergency.
The concentration of heparin used to lock hemodialysis catheters ranges from 1000 to 10,000 units/mL. Researchers have demonstrated that using 1,000 units/mL reduces the incidence of post-insertion bleeding but may be associated with an increased need for recombinant tissue plasminogen activator (tPA) to maintain patency. A flush of 1000 units heparin/mL or a solution of 4% sodium citrate in the amount of the dwell volume of each lumen has been recommended (Moran & Ash, 2010). Heparin is most often used because sodium citrate has not been as commercially available in the preparation needed. To prevent systemic anticoagulation and subsequent bleeding, be sure to aspirate the heparin from the lumens before use.
Infusion Systems
Nurses administering infusion therapies need to understand how infusion systems work. This knowledge ensures that the patient can benefit from a particular system’s advantages while minimizing any potential complications.
Containers
Infusion containers are made of glass or plastic. Glass bottles were the original fluid container to be mass produced. They are easily sterilized, and it is easy to read the amount of fluid remaining in the bottle. Also, glass is inert and so cannot interact with some drugs like plastic can. However, glass bottles are heavy and cannot easily be used in many situations, such as patient transport during emergencies. These containers require an air vent for fluids to flow freely from them. The most common method is to use an administration set with a special filtered vent. Some bottles may have a straw tube open to the room air through the rubber stopper in the bottle and extending to above the level of the fluid. Bottles with a venting straw do not have a barrier to prevent contaminants in the air from entering the fluid.
Plastic containers are considered closed systems because they do not rely on outside air to allow the fluid to infuse. Instead, atmospheric pressure pushes against the flexible sides of the container, allowing the fluid to flow by gravity. For this reason, plastic containers do not require vented administration sets. These containers are lightweight, resistant to breaking, easier to store, and easy to use in emergency conditions. Therefore they are used more frequently than glass containers.
All plastic containers were commonly made of polyvinyl chloride (PVC). To increase flexibility and strength, PVC required the addition of a plasticizer, such as di-2-ethylhexyl-phthalate or DEHP. Concern has been growing in the past few years over the exposure of patients to this chemical because it can leach from the plastic fluid container or tubing and infuse into the patient with the IV fluid or medication. The Food and Drug Administration (FDA) has determined that there is little to no risk posed to most patients by exposure to the amount of DEHP released from IV bags with infusion of crystalloids or drugs. However, there is concern about the buildup of chemical exposure from many sources over a lifetime and specifically the potential effects of DEHP on the development of the male reproductive system. Therefore many hospitals are using PVC-free or DEHP-free IV bags, especially for high-risk groups such as neonates and children (Food and Drug Administration [FDA], 2009).
One disadvantage of removing DEHP from the plastic bags is that the bags are less pliable and more prone to rupture. Some institutions do not allow these bags to be sent through a pneumatic tube system because the pressure exerted has caused the bags to rupture during transport.
A problem with plastic containers is that they are not compatible with insulin, nitroglycerin, lorazepam (Ativan), fat emulsions, and lipid-based drugs. Nitroglycerin and insulin adhere to the walls of the PVC container, making it impossible to know exactly how much medication the patient is receiving. There is evidence that a priming volume of 20 mL of insulin solution is required to minimize the effect of insulin absorption losses to the plastics in IV lines (Goldberg et al., 2006).
Another concern with plastic bags is the accuracy of reading the amount of fluid remaining in the container. The middle graduations have been shown to be 10% above or below the actual amount of fluid, but the first and last markings could be inaccurate by as much as 40% (Perucca, 2010).
Semi-rigid containers are made of plastic but offer some of the benefits of glass, such as the absence of plasticizers and drug incompatibility. These containers are lightweight and unbreakable but are more bulky and less flexible. They also require an air vent to allow the fluid to flow freely.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree