CHAPTER 69 Immunosuppressive drugs inhibit immune responses. They have two principal applications: (1) prevention of organ rejection in transplant recipients, and (2) treatment of autoimmune disorders (eg, rheumatoid arthritis, systemic lupus erythematosus). At the doses required to suppress allograft rejection, almost all of these drugs are toxic. Two toxicities are of particular concern: (1) increased risk of infection and (2) increased risk of neoplasms. Furthermore, because allograft recipients must take immunosuppressants for life, the risk of toxicity continues lifelong. Sites of action of immunosuppressants are summarized in Figure 69–1. Cyclosporine [Sandimmune, Gengraf, Neoral] is a powerful immunosuppressant and the drug of choice for preventing organ rejection in recipients of an allogenic transplant.* Major adverse effects are nephrotoxicity and increased risk of infection. Cyclosporine is used primarily to prevent rejection of allogenic kidney, liver, and heart transplants. A glucocorticoid (prednisone) is usually given concurrently. Azathioprine, tacrolimus, or sirolimus may be given as well. Additional indications are psoriasis (see Chapter 105) and rheumatoid arthritis (see Chapter 73). Systemic tacrolimus is approved for prophylaxis of organ rejection in patients receiving liver, kidney, or heart transplants. Concurrent use of glucocorticoids is recommended (along with azathioprine or mycophenolate mofetil for heart or kidney recipients). Compared with patients receiving cyclosporine, those receiving tacrolimus experience fewer episodes of acute transplant rejection, but twice as many patients discontinue the drug because of toxicity. Tacrolimus is under investigation for use in patients receiving bone marrow, pancreas, and small bowel transplants. As discussed in Chapter 105, tacrolimus is also used for topical therapy of atopic dermatitis.
Immunosuppressants
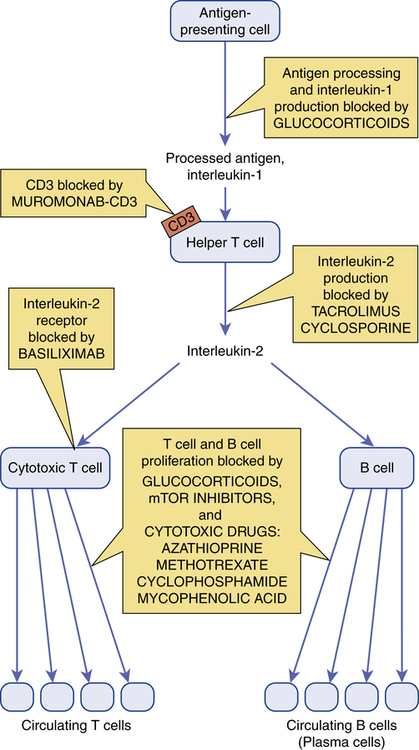
 Sites of action of immunosuppressant drugs.
Sites of action of immunosuppressant drugs.
Calcineurin inhibitors
Cyclosporine
Therapeutic uses
Tacrolimus
Therapeutic use.
Get Clinical Tree app for offline access

Immunosuppressants
Get Clinical Tree app for offline access


