CHAPTER 63 In this chapter, we discuss infertility in two stages. First, we discuss the underlying causes of reproductive dysfunction. Second, we discuss the fertility-promoting drugs. As preparation to study these agents, you should review Chapter 61 for information on the menstrual cycle and information on the biosynthesis and physiologic and pharmacologic effects of estrogens and progestins. Pay special attention to the roles of gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH). • Weight loss, through exercise and diet, can reduce insulin and androgen levels, improve insulin sensitivity, restore menstruation and ovulation, and increase pregnancy rates. • Clomiphene [Clomid, Milophene, Serophene] is considered a first-line drug for inducing ovulation. It may be used alone or in combination with metformin. • Metformin [Glucophage, others], a drug for type 2 diabetes, increases insulin sensitivity and decreases insulin levels, which, through an indirect mechanism, lowers androgen levels. The net result is improved glucose tolerance, improved ovulation, and increased pregnancy rates. • Pioglitazone [Actos], another drug for type 2 diabetes, acts like metformin, causing an increase in insulin sensitivity, and a decrease in insulin levels and androgen levels. However, pioglitazone can harm the fetus, and hence should not be used by women trying to become pregnant. • Oral contraceptives can restore regular periods and reduce acne and hirsutism, but obviously won’t improve fertility. • Spironolactone has antiandrogenic actions, and can thereby decrease hirsutism and acne. The drug can harm the fetus, and hence must not be used during pregnancy. A few males may be incapable of spermatogenesis owing to insufficient gonadotropin secretion. In these rare cases, drugs may help. If the gonadotropin deficiency is only partial, sperm counts can be increased using hCG (alone or in combination with menotropins). If the deficiency is severe, treatment with androgens is required (see Chapter 65). If therapy with hCG and menotropins is intended, the patient should be informed that treatment will be both prolonged (3 to 4 years) and expensive. The term controlled ovarian stimulation refers to the use of drugs to facilitate follicular maturation and ovulation. Following ovulation, fertilization can be accomplished either naturally (through sexual intercourse) or through assisted reproduction technology (eg, in vitro fertilization). Of the drugs discussed below, six are used to promote follicular maturation, two are used to stimulate ovulation, and two are used to prevent premature stimulation of ovulation by endogenous hormones (Table 63–1). TABLE 63–1 Drugs for Controlled Ovarian Stimulation
Drug therapy of infertility
Infertility: causes and treatment strategies
Female infertility
Polycystic ovary syndrome
Male infertility
Hypogonadotropic hypogonadism
Drugs used to treat female infertility
Drugs for controlled ovarian stimulation

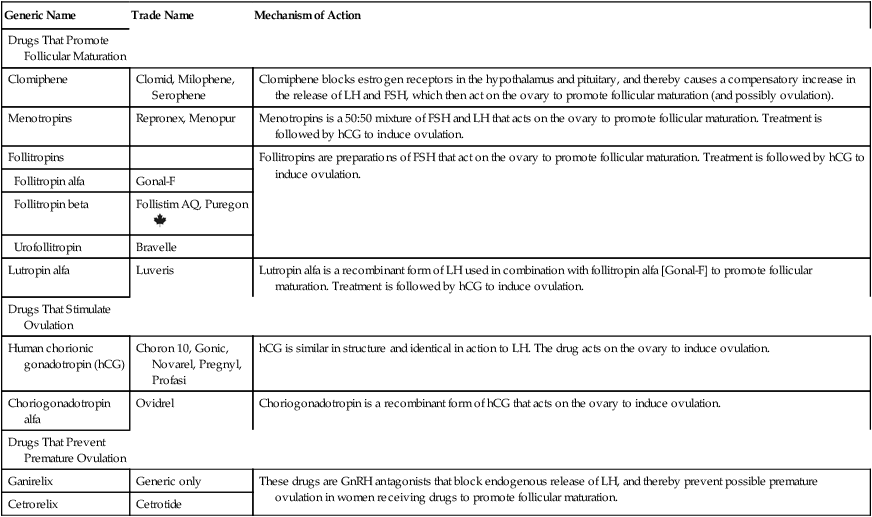
Generic Name
Trade Name
Mechanism of Action
Drugs That Promote Follicular Maturation
Clomiphene
Clomid, Milophene, Serophene
Clomiphene blocks estrogen receptors in the hypothalamus and pituitary, and thereby causes a compensatory increase in the release of LH and FSH, which then act on the ovary to promote follicular maturation (and possibly ovulation).
Menotropins
Repronex, Menopur
Menotropins is a 50:50 mixture of FSH and LH that acts on the ovary to promote follicular maturation. Treatment is followed by hCG to induce ovulation.
Follitropins
Follitropins are preparations of FSH that act on the ovary to promote follicular maturation. Treatment is followed by hCG to induce ovulation.
Follitropin alfa
Gonal-F
Follitropin beta
Follistim AQ, Puregon ![]()
Urofollitropin
Bravelle
Lutropin alfa
Luveris
Lutropin alfa is a recombinant form of LH used in combination with follitropin alfa [Gonal-F] to promote follicular maturation. Treatment is followed by hCG to induce ovulation.
Drugs That Stimulate Ovulation
Human chorionic gonadotropin (hCG)
Choron 10, Gonic, Novarel, Pregnyl, Profasi
hCG is similar in structure and identical in action to LH. The drug acts on the ovary to induce ovulation.
Choriogonadotropin alfa
Ovidrel
Choriogonadotropin is a recombinant form of hCG that acts on the ovary to induce ovulation.
Drugs That Prevent Premature Ovulation
Ganirelix
Generic only
These drugs are GnRH antagonists that block endogenous release of LH, and thereby prevent possible premature ovulation in women receiving drugs to promote follicular maturation.
Cetrorelix
Cetrotide