Chapter 6 On completion of this chapter, the reader will be able to: • Explain the key concepts of basic human genetics. • Explore how recent advances in genetics have changed the field of health care. • Discuss key findings and the ethical, legal, and social implications of the Human Genome Project. • Describe expanded roles for nurses in genetics and genetic counseling. • Identify genetic disorders commonly tested for in maternity and newborn nursing. • Discuss the current status of gene therapy. • Summarize the process of fertilization. • Describe the development, structure, and functions of the placenta. • Describe the composition and functions of the amniotic fluid. • Identify three organs or tissues arising from each of the three primary germ layers. • Summarize the significant changes in growth and development of the embryo and fetus. • Identify the potential effects of teratogens during vulnerable periods of embryonic and fetal development. Recent advances in molecular biology and genomics have revolutionized the field of health care by providing the tools needed to determine the hereditary component of many diseases as well as improve our ability to predict susceptibility to disease, onset and progression of disease, and response to medications (Guttmacher, McGuire, Ponder, et al., 2010). This increase in genetic knowledge has resulted in a gradual shift from genetics to genomics. Genetics is the study of individual genes and their effect on relatively rare single-gene disorders, whereas genomics is the study of all the genes in the human genome together, including their interactions with each other, the environment, and the influence of other psychosocial factors and cultural factors. Genes are basic physical units of inheritance that are passed from parents to offspring and contain the information needed to specify traits. The genome is the entire set of genetic instructions found in each cell. For these and other definitions of genetic terms, visit the Talking Glossary of Genetic Terms (www.genome.gov/Glossary). Genetic services are rapidly becoming an integral part of routine health care as a result of: • Growing public interest in personalized genomic information (information about much or all of a person’s genome) • Increasing development of practice guidelines • Mounting commercial pressures • Ever-increasing opportunities for individuals, families, and communities to participate in the direction and design of their genomic health care (Guttmacher, McGuire, Ponder, et al., 2010) Moreover, many individuals and families have participated in direct-to-consumer genetic testing (testing marketed directly to consumers through television, print advertisements, and websites for companies such as DNA Direct [www.dnadirect.com/web], 23 and Me [www.23andme.com], and DeCODEme [www.decodeme.com]). Although much of the information provided by direct-to-consumer testing companies is recreational (e.g., ancestry information, information about type of ear wax, bitter taste perception), some of it is health related and could be interpreted as diagnosis (Evans and Green, 2009). Because of this, direct-to-consumer testing that is provided without the involvement of competent health care professionals may be not only unhelpful but even harmful (Guttmacher, McGuire, Ponder, et al., 2010; McGuire and Burke, 2010). • Preconception counseling and testing • Neonatal genetic screening and testing • Palliative care for infants with life-threatening genetic conditions and their families • The identification and care of individuals with genetic conditions and their families • The care of women with genetic conditions who require specialized care during pregnancy, such as women with congenital heart disease, cystic fibrosis, and factor V Leiden. Nearly 50 organizations, including the Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN) and the National Association of Neonatal Nurses (NANN), have endorsed the Essentials of Genetic and Genomic Nursing: Competencies, Curricula Guidelines, and Outcome Indicators (ed 2) (Consensus Panel on Genetic/Genomic Nursing Competencies, 2009). The competencies in the document reflect the minimum amount of genetic and genomic competency expected of all nurses. The competencies are not intended to replace or recreate current standards of practice. The document is available at www.genome.gov/Pages/Careers/HealthProfessionalEducation/geneticscompetency.pdf. Some of the competencies most relevant to nurses in maternity nursing include: • Constructs a pedigree from collected family history information using standardized symbols and terminology • Develops a plan of care that incorporates genetic and genomic assessment information • Provides patients with credible, accurate, appropriate, and current genetic and genomic information, resources, services, and/or technologies that facilitate decision making • Recognizes when one’s own attitudes and values related to genetic and genomic science may affect care provided to patients • Facilitates referrals for specialized genetic and genomic services for patients as needed • Evaluates impact and effectiveness of genetic and genomic technology, information, interventions, and treatments on patients’ outcome The Human Genome Project was a publicly funded international effort coordinated by the National Institutes of Health (NIH) and the U.S. Department of Energy (www.doegenomes.org). When the Human Genome Project was initiated in 1990, the ultimate goal of the project was to map the human genome (the complete set of genetic instructions in the nucleus of each human cell) by 2005. Considering that the human genome consists of approximately 3 billion base pairs of DNA, many people regarded this as an impossible task. However, by 2003 a substantially complete version of the human genome was announced. A more recent effort by the National Human Genome Research Institute (NHGRI) called the Encyclopedia of DNA Elements, or ENCODE, was organized to identify the genome’s functional elements. By 2012 researchers “linked more than 80% of the human genome sequence to a specific biological function and mapped more than 4 million regulatory regions where proteins specifically interact with the DNA” (ENCODE, 2012). This made clearer the active genome in which genes are turned on and off by proteins using sites that may be at a great distance from the genes. Identification of regulatory regions will help explain different functions of different types of cells (www.genome.gov/pfv.cfm?pageID=27549810). Completion of the Human Genome Project and the resultant identification of the inherited causes for many diseases have created a renewed interest in family history. Although it is easy to be impressed by the 1900 genetic tests currently available, family history will most likely continue to be the single most cost-effective piece of genetic information. A complete three-generation family history that includes ethnicity information concerning both sides of the family is the best genetic “test” applicable to preconception care. When nurses and other clinicians conduct a family history, they can gain not only valuable information about the structure of the family and diseases that affect various individuals in the family but also a rich understanding of family relationships, social context, occupations, lifestyle, and health habits. The process of collecting this information often facilitates the development of a relationship between the patient/family and the clinician. In 2004 the United States Department of Health and Human Services (USDHHS) launched the Family History Initiative by designating Thanksgiving Day as National Family History Day. The U.S. Surgeon General encouraged families to use their family gatherings as a time to talk about and collect important family health history. A number of family history tools are available free of charge online. One of the most widely used is the My Family Health Portrait (https://familyhistory.hhs.gov). Another family health history tool—Does it run in the family?—was developed by the Genetic Alliance (www.doesitruninthefamily.org). Initial efforts to sequence and analyze the human genome have proven invaluable in the identification of genes involved in disease and in the development of genetic tests. Hundreds of genes involved in diseases such as breast cancer, colorectal cancer, Alzheimer’s disease, and cystic fibrosis (CF) have been identified. The number of commercially available genetic tests continues to increase and can be found on GeneTests, a publicly funded genetics information resource for clinicians (www.ncbi.nlm.nih.gov/sites/GeneTests/). • Maternal serum screening (a blood test used to see if a pregnant woman is at increased risk for carrying a fetus with a neural tube defect or chromosomal abnormalities such as Down syndrome, trisomy 18, and trisomy 13) • Fetal ultrasound or sonogram (an imaging technique using high-frequency sound waves to produce images of the fetus inside the uterus) • Invasive procedures (amniocentesis and chorionic villus sampling) (See Chapter 10 for discussion of these tests.) Other tests are carrier screening tests used to identify individuals who have a gene mutation for a genetic condition but do not show symptoms of the condition because it is an autosomal recessive condition (e.g., CF, sickle cell disease, Tay-Sachs disease). In addition to using genetic tests for single-gene disorders in patients with clinical symptoms or with a family history of a genetic disease, genetic tests are being used for population-based screening. For example, newborn screening for phenylketonuria (PKU) and other inborn errors of metabolism (IEMs) has been going on in the United States and many other countries for decades (Guttmacher, McGuire, Ponder, et al., 2010). Initially, state-mandated newborn screening in the United States was concerned with only a few conditions. With the advent of tandem mass spectrometry, the number of conditions tested for during newborn screening grew rapidly. Currently, most states use blood spots collected from newborns to test for at least 30 different metabolic and genetic diseases. The conditions most commonly tested for are PKU, congenital hypothyroidism, galactosemia, and sickle cell disease and other hemoglobinopathies. A complete list of conditions tested for in each state is available on the National Newborn Screening and Genetics Resource website (genes-r-us.uthscsa.edu). Genetic tests are also used to determine paternity, identify victims of war and other tragedies, and profile criminals (www.genetests.org). One of most promising clinical applications of the Human Genome Project has been pharmacogenomic testing (the use of genetic information to individualize drug therapy). Associations between genetic variation and drug effect have been observed for a number of commonly used drugs. One of these drugs is warfarin, an anticoagulant commonly used to reduce the risk for thromboembolic events in patients with a history of deep vein thrombosis, pulmonary embolism, myocardial infarction, or atrial fibrillation (Meckley, Gudgeon, Anderson, et al., 2010). There is mounting evidence that genotype-guided warfarin dosing may not only help reduce the serious adverse drug reactions commonly associated with warfarin but also increase dosing accuracy, shorten the time to dose stabilization, and help identify individuals who may require more frequent monitoring. • Privacy and fairness in the use and interpretation of genetic information • Clinical integration of new genetics technologies • Issues surrounding genetics research, such as possible discrimination and stigmatization • Education for professionals and the general public about genetics, genetics health care, and ELSI of human genome research Both ELSI programs have excellent websites that include much educational information, as well as links to other informative sites (www.genome.gov/10001618; www.ornl.gov/sci/techresources/Human_Genome/elsi/elsi.shtml). Chromosomal abnormalities are a major cause of reproductive loss, congenital problems, and gynecologic disorders. The incidence of abnormalities is approximately 0.6% in newborns, 6% in stillbirths, and 60% in spontaneous abortions (Martin, 2008). Errors resulting in chromosomal abnormalities can occur in mitosis (cell division occurring in somatic cells that results in two identical daughter cells containing a diploid number of chromosomes) or meiosis (division of a sex cell into two and four haploid cells). These errors can occur in either the autosomes or the sex chromosomes. Even without the presence of obvious structural malformations, small deviations in chromosomes can cause problems in fetal development. The pictorial analysis of the number, form, and size of an individual’s chromosomes is known as a karyotype. Cells from any nucleated, replicating body tissue (not red blood cells, nerves, or muscles) can be used. The most commonly used tissues are white blood cells and fetal cells in amniotic fluid. The cells are grown in a culture and arrested when they are in metaphase (during metaphase, the chromosomes are condensed and visible with a light microscope), and then the cells are dropped onto a slide. This breaks the cell membranes and spreads the chromosomes, making them easier to visualize. Next, the cells are stained with special stains (e.g., Giemsa stain) that create striping or “banding” patterns. These patterns aid in the analysis because they are consistent from person to person. Once the chromosome spreads are photographed or scanned by a computer, they are cut out and arranged in a specific numeric order according to their length and shape. The chromosomes are numbered from largest to smallest, 1 to 22, and the sex chromosomes are designated by the letter X or Y. Each chromosome is divided into two “arms” designated by p (short arm) and q (long arm). A female karyotype is designated as 46,XX and a male karyotype is designated as 46,XY. Fig. 6-1 illustrates the chromosomes in a body cell and a karyotype. The most common trisomy abnormality is Down syndrome (DS). Approximately one in every 691 newborns has DS; there are over 400,000 individuals with DS living in the United States (www.ndss.org). Ninety-five percent of individuals with DS have trisomy 21 (nondisjunction) or an extra chromosome 21 (47,XX+21, female with DS; or 47,XY+21, male with DS). Another type of DS, translocation, occurs when extra chromosome 21 material is present in every cell of the individual but it is attached to another chromosome. In the third type of DS, mosaicism, extra chromosome 21 material is found in some but not all of the cells. Although the risk for having a child with DS increases with maternal age (incidence is approximately 1 in 1200 for a 25-year-old woman; 1 in 350 for a 35-year-old woman; and 1 in 30 for a 45-year-old woman), children with Down syndrome can be born to mothers of any age (www.ndss.org). Eighty percent of children with Down syndrome are born to mothers younger than 35 years. The risk for a mother having a second child with Down syndrome is about 1% when the cause of the Down syndrome is trisomy 21. The most common deviation in males is Klinefelter syndrome, or trisomy XXY. The affected male has poorly developed secondary sexual characteristics and small testes. He is infertile, usually tall, and effeminate and may be slow to learn (www.genetic.org). Males who have mosaic Klinefelter syndrome may be fertile. If a single gene controls a particular trait or disorder, its pattern of inheritance is referred to as unifactorial mendelian or single-gene inheritance. The number of single-gene disorders far exceeds the number of chromosomal abnormalities. Potential patterns of inheritance for single-gene disorders include autosomal dominant, autosomal recessive, and X-linked dominant and recessive modes of inheritance (Fig. 6-2). Autosomal dominant inheritance disorders are those in which only one copy of a variant allele is needed for phenotypic expression. The variant allele may be a result of a mutation—a spontaneous and permanent change in the normal gene structure in which case the disorder occurs for the first time in the family. Usually an affected individual comes from multiple generations having the disorder. An affected parent who is heterozygous for the trait has a 50% chance of passing the variant allele to each offspring (see Fig. 6-2, B and C). There is a vertical pattern of inheritance (i.e., there is no skipping of generations; if an individual has an autosomal dominant disorder such as HD, so must one of his or her parents). Males and females are equally affected. Autosomal recessive inheritance disorders are those in which both genes of a pair associated with the disorder must be abnormal for the disorder to be expressed. Heterozygous individuals have only one variant allele and are unaffected clinically because their normal gene overshadows the variant allele. They are known as carriers of the recessive trait. Because these recessive traits are inherited by generations of the same family, an increased incidence of the disorder occurs in consanguineous matings (closely related parents). For the trait to be expressed, two carriers must each contribute a variant allele to the offspring (see Fig. 6-2, C). The chance of the trait occurring in each child is 25%. A clinically normal offspring may be a carrier of the gene. Autosomal recessive disorders have a horizontal pattern of inheritance rather than the vertical pattern seen with autosomal dominant disorders. That is, autosomal recessive disorders are usually observed in one or more siblings but not in earlier generations. Males and females are equally affected. Most inborn errors of metabolism (IEMs), such as phenylketonuria, galactosemia, maple syrup urine disease, Tay-Sachs disease, sickle cell anemia, and cystic fibrosis, are autosomal recessive inherited disorders. More than 350 inborn errors of metabolism have been recognized (Jorde, Carey, and Bamshad, 2010). Individually, IEMs are relatively rare, but collectively, they are common (1 in 5000 live births). Most IEMs are inherited in an autosomal recessive pattern. IEMs occur when a gene mutation reduces the efficiency of encoded enzymes to a level at which normal metabolism cannot occur. Defective enzyme action interrupts the normal series of chemical reactions from the affected point onward. The result may be an accumulation of a damaging product, such as phenylalanine in PKU, or the absence of a necessary product, such as the lack of melanin in albinism caused by lack of tyrosinase. Diagnostic and carrier testing is available for a growing number of IEMs. In addition, many states in the United States have started screening for specific IEMs as part of their expanded newborn screening programs using tandem mass spectrometry. However, many of the deaths caused by IEMs are the result of enzyme variants not currently screened for in many of the newborn screening programs (Jorde, Carey, and Bamshad, 2010). (See discussion of IEMs in Chapter 25.) It is standard practice in obstetrics to determine whether a heritable disorder exists in a couple or in anyone in either of their families. The goal of screening is to detect or define risk for disease in low risk populations and identify those for whom diagnostic testing may be appropriate. A nurse can obtain a genetics history using a questionnaire or checklist such as the one in Fig. 6-3.
Genetics, Conception, and Fetal Development
Genetics
Nursing Expertise in Genetics and Genomics
Essential Competencies in Genetics and Genomics for All Nurses
Human Genome Project and Implications for Clinical Practice
Importance of Family History
Gene Identification and Testing
Pharmacogenomics
Ethical, Legal, and Social Implications
Chromosomal Abnormalities
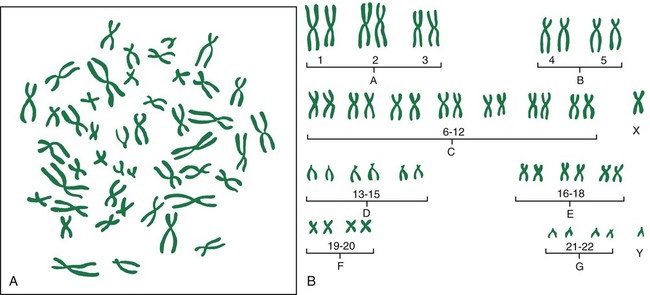
Autosomal Abnormalities
Abnormalities of Chromosome Number.
Sex Chromosome Abnormalities
Patterns of Genetic Transmission
Unifactorial Inheritance
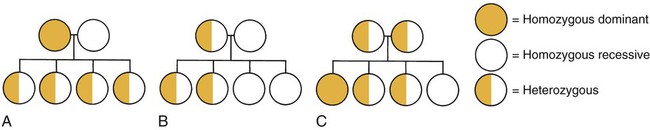
Autosomal Dominant Inheritance.
Autosomal Recessive Inheritance.
Inborn Errors of Metabolism.
Genetic Counseling

Genetics, Conception, and Fetal Development
Get Clinical Tree app for offline access



