(gan-SYE-kloh-veer SO-dee-um)
Cytovene IV
Antiviral
pH 9 to 11
Usual dose
Adequate hydration and specific testing required; see Monitor.
CMV retinitis:
5 mg/kg of body weight every 12 hours for 14 to 21 days. Begin a maintenance dose the next day (Day 15 to 22) of 5 mg/kg daily for 7 days each week or 6 mg/kg daily for 5 days each week. See Precautions/Monitor. After IV induction and when retinitis is stable, Cytovene capsules may be used for maintenance therapy; see Precautions. If retinitis progresses during the maintenance regimen, initiate the twice-daily program again. Do not exceed recommended dose or infusion rate. Larger doses or increased rates of infusion have resulted in increased toxicity. For patients who have relapsed after induction and reinduction monotherapy with either ganciclovir or foscarnet, practitioners may consider an unlabeled combination therapy, which adds the alternate drug to the regimen. Information on this combination regimen is available from Roche and in the Clinical Trials section of the foscarnet sodium prescribing information.
Prevention of CMV disease in transplant recipients:
5 mg/kg every 12 hours for 7 to 14 days. Follow with maintenance regimen as outlined in CMV retinitis. Length of treatment based on immunosuppression degree and duration; 3 to 4 months or longer is common. CMV disease may occur if treatment is stopped prematurely.
Prevention of CMV disease in HIV-infected adolescents and adults (unlabeled):
5 to 6 mg/kg/dose for 5 to 7 days each week. Cytovene capsules may be used.
Pediatric dose
Safety for use in pediatric patients under 12 years of age not established. ■ Use extreme caution; long-term carcinogenicity and reproductive toxicity are probable. Benefit must outweigh risks. See Indications and Maternal/Child.
CMV retinitis in pediatric patients over 3 months of age (unlabeled):
2.5 mg/kg every 8 hours for 14 to 21 days followed by a maintenance dose of 6 to 6.5 mg/kg/day was used during clinical trials. When retinitis progressed, the adult dosing regimen for induction and maintenance was followed. Another source suggests the adult dose regimen listed in Usual Dose.
Prevention of CMV disease in transplant recipients (unlabeled):
Same as adults; see Usual Dose.
Prevention of CMV disease in HIV-infected individuals (unlabeled):
5 mg/kg/dose IV daily.
Dose adjustments
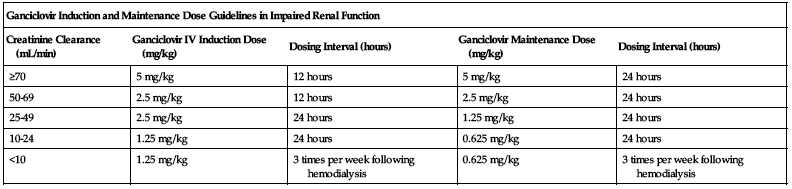
Dose selection should be cautious in the elderly. Reduced doses may be indicated based on the potential for decreased organ function and concomitant disease or drug therapy. Assess renal function before administration to elderly patients and adjust dose appropriately; see Elderly. ■ Withhold dose if absolute neutrophil count (ANC) less than 500 cells/mm3 or platelets less than 25,000 cells/mm3. ■ See Drug/Lab Interactions. ■ With impaired renal function, reduce dose according to the following chart.
| Ganciclovir Induction and Maintenance Dose Guidelines in Impaired Renal Function | ||||
| Creatinine Clearance (mL/min) | Ganciclovir IV Induction Dose (mg/kg) | Dosing Interval (hours) | Ganciclovir Maintenance Dose (mg/kg) | Dosing Interval (hours) |
| ≥70 | 5 mg/kg | 12 hours | 5 mg/kg | 24 hours |
| 50-69 | 2.5 mg/kg | 12 hours | 2.5 mg/kg | 24 hours |
| 25-49 | 2.5 mg/kg | 24 hours | 1.25 mg/kg | 24 hours |
| 10-24 | 1.25 mg/kg | 24 hours | 0.625 mg/kg | 24 hours |
| <10 | 1.25 mg/kg | 3 times per week following hemodialysis | 0.625 mg/kg | 3 times per week following hemodialysis |
Dilution
Specific techniques required; see precautions.
Initially dissolve the 500-mg vial with 10 mL SWFI (50 mg/mL). Do not use bacteriostatic water containing parabens; will cause precipitation. Shake well to dissolve completely. Discard if particulate matter or discoloration observed. Withdraw desired dose and further dilute with NS, D5W, Ringer’s, or LR to provide a concentration less than 10 mg/mL (70-kg adult at 5 mg/kg equals 350 mg; dissolved in 100 mL of solution equals 3.5 mg/mL).
Filters:
Use not required by manufacturer; however, use of a filter would not have an adverse effect.
Storage:
Store unopened vials below 40° C (104° F). Reconstituted solution in vial stable at CRT for 12 hours. Do not refrigerate. Solution fully diluted for administration must be refrigerated and used within 24 hours to reduce incidence of bacterial contamination. Stable for 14 days refrigerated at 5° C (41° F) if prepared in PVC bags and reconstituted with SWFI and further diluted with NS.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Because of physical incompatibilities, ganciclovir sodium and foscarnet sodium must never be mixed.
One source suggests the following compatibilities:
Y-site:
Allopurinol (Aloprim), amphotericin B cholesteryl (Amphotec), anidulafungin (Eraxis), caspofungin (Cancidas), cisatracurium (Nimbex), docetaxel (Taxotere), doxorubicin liposomal (Doxil), enalaprilat (Vasotec IV), etoposide phosphate (Etopophos), filgrastim (Neupogen), fluconazole (Diflucan), granisetron (Kytril), linezolid (Zyvox), melphalan (Alkeran), paclitaxel (Taxol), pemetrexed (Alimta), propofol (Diprivan), remifentanil (Ultiva), teniposide (Vumon), thiotepa.
Rate of administration
A single dose must be administered at a constant rate over 1 hour as an infusion. Use of an infusion pump or microdrip (60 gtt/mL) recommended. Do not give by rapid or bolus IV injection. Excessive plasma levels and toxicity will occur with too-rapid rate of injection. Advisable to clear tubing with NS before and after administration through Y-tube or three-way stopcock.
Actions
An antiviral agent that inhibits DNA synthesis and stops cytomegalovirus (CMV) from multiplying. Does not destroy existing viruses but stops them from reproducing and invading healthy cells. May allow a weakened immune system to defend the body against the CMV infection. May also be inhibitory against herpes simplex virus 1 and 2, Epstein-Barr virus, and varicella zoster virus, but clinical studies have not been done. Onset of action is prompt, and therapeutic levels are maintained for 3 to 6 hours with some drug remaining 11 hours after infusion. Widely distributed in tissues and body fluids. Half-life is 2.6 to 4.4 hours. Crosses the placental barrier. Suspected to be secreted in breast milk. Approximately 90% excreted unchanged in urine in patients with normal renal function.
Indications and uses
Treatment of CMV retinitis in immunocompromised individuals, including patients with AIDS. ■ CMV disease prevention in at-risk transplant patients. ■ Safety and effectiveness for use in congenital or neonatal CMV disease, treatment of established CMV disease other than retinitis, or nonimmunocompromised individuals not established. Use should be limited to treatment of conditions listed above. ■ Now available as an ophthalmic surgical aid (intravitreal implant) to treat CMV retinitis. ■ Ganciclovir (Cytovene) capsules are used for prevention of CMV retinitis in at-risk patients with advanced HIV infection and in the prevention of CMV disease in solid-organ transplant recipients; see Precautions. Valganciclovir (Valcyte) is an oral drug recently approved for the treatment of CMV retinitis, and it is also being used for maintenance.
Unlabeled uses:
Treatment of other CMV infections (e.g., gastroenteritis, hepatitis, pneumonitis) in immunocompromised patients. ■ Treatment of polyradiculopathy caused by CMV infections. ■ Combination therapy (ganciclovir and foscarnet) to treat progressive retinitis refractory to single therapy.
Contraindications
Hypersensitivity to ganciclovir or acyclovir; patients with a neutrophil count less than 500 cells/mm3 or a platelet count less than 25,000 cells/mm3; patients receiving zidovudine (AZT, Retrovir) because both drugs cause granulocytopenia.
Precautions
A nucleoside analog; follow guidelines for handling and disposal of cytotoxic agents. See Appendix A, p. 1331. ■ For IV use only; IM or SC administration will cause severe tissue irritation. ■ Hematologic toxicity (anemia, granulocytopenia, thrombocytopenia) is common; see Monitor. ■ Use with caution in patients with pre-existing cytopenias or a history of cytopenic reactions to other drugs, chemicals, or irradiation. ■ Ganciclovir is not a cure for CMV infections. Maintenance therapy is almost always necessary to prevent relapse in patients with AIDS. ■ Resistance has been reported to develop. May be higher in patients treated for a prolonged period. ■ Cidofovir is also an agent for treatment of CMV retinitis. ■ Risk of a more rapid rate of disease progression is increased with oral ganciclovir. Use in maintenance recommended only if benefits of avoiding daily IV infusions outweigh risk.
Monitor:
Confirm diagnosis of CMV retinitis by indirect ophthalmoscopy. Diagnosis may be supported by cultures of CMV (e.g., urine, blood, throat); negative culture does not rule out CMV retinitis. ■ Continue ophthalmologic exams during induction and maintenance treatment to monitor CMV status. ■ CBC with differential and platelet counts, SCr, and CrCl are required before treatment is initiated. Monitor CBC and platelet counts frequently, especially in patients with previous leukopenia from ganciclovir or other nucleoside analogs or those with neutrophils less than 1,000 cells/mm3 at beginning of treatment and in patients undergoing hemodialysis. Withhold dose if absolute neutrophil count (ANC) less than 500 cells/mm3 or platelets less than 25,000 cells/mm3. Monitor SCr or CrCl every 2 weeks. ■ Maintain adequate hydration and urine flow before and during infusion. ■ Phlebitis or pain may occur at site of infusion; confirm patency of vein and use small needles and large veins to ensure adequate blood flow for rapid dilution and distribution. ■ Granulocytopenia usually occurs within 14 days but may occur at any time; recovery should begin within 3 to 7 days of discontinuing ganciclovir. ■ Consider the possibility of viral resistance if retinitis does not show significant improvement with treatment. ■ See Drug/Lab Interactions.
Patient education:
Must use effective birth control throughout treatment. Men should continue barrier contraception for at least 90 days. ■ Not a cure; retinitis may still progress. Frequent ophthalmoscopic examinations important. ■ Cooperation for close monitoring of blood cell counts is imperative to control side effects (e.g., anemia, neutropenia, thrombocytopenia). ■ Report any unexpected side effects promptly (e.g., chills, fever, unusual bleeding or bruising). ■ Patients with AIDS receiving zidovudine (AZT, Retrovir) may not tolerate ganciclovir. ■ High frequency of impaired renal function increased with concomitant use of nephrotoxic agents (e.g., cyclosporine, amphotericin); high risk for transplant recipients.
Maternal/child:
Category C: avoid pregnancy. A potential carcinogen. Teratogenic and embryotoxic; has caused aspermatogenesis and will cause birth defects. Do not use during pregnancy unless risk is justified. May cause temporary or permanent infertility in men and women. ■ Discontinue nursing during treatment; minimum interval required before resuming breast-feeding is unknown. ■ Use extreme caution in pediatric patients under 12 years of age. Long-term carcinogenicity and reproductive toxicity are probable. Benefit must outweigh risks.
Elderly:
Dose selection should be cautious; see Dose Adjustments. Monitor renal function during therapy and adjust dose as indicated.
Drug/lab interactions
Because of physical incompatibilities, ganciclovir and foscarnet must never be mixed. ■ Additive toxicity may occur with concomitant use of other drugs that inhibit replication of rapidly dividing cell populations (e.g., dapsone, pentamidine, flucytosine [Ancobon], vincristine, vinblastine, doxorubicin [Adriamycin], amphotericin B [conventional], sulfamethoxazole/trimethoprim [Bactrim]). ■ May cause severe anemia and neutropenia with zidovudine (AZT, Retrovir). Combination used in patients with AIDS is rarely tolerated. ■ Concurrent treatment with didanosine (Videx) may cause increased didanosine levels. ■ May cause seizures with imipenem-cilastatin (Primaxin). ■ Potentiated by probenecid and other drugs that may reduce renal clearance; will increase toxicity. ■ Impaired renal function may be markedly increased with other nephrotoxic agents (e.g., cyclosporine, amphotericin B). ■ Concurrent or consecutive use with other bone marrow suppressants (e.g., antineoplastics, amphotericin B, zidovudine and/or radiation therapy) may produce additive bone marrow suppression. ■ Drug interaction studies with drugs commonly used in transplant recipients have not been conducted. ■ See Contraindications.
Side effects
Anemia, leukopenia, and thrombocytopenia are most common and are generally reversible if treatment is discontinued. Abdominal pain; anorexia; chills; diarrhea; fever; infection; nausea; neuropathy; pain, infection, and sepsis at injection site; phlebitis; pruritus; rash; retinal detachment; sepsis; sweating; and vomiting occur in some patients. Abnormal kidney function and/or failure, abnormal vision, alopecia, anxiety, arthralgia, asthenia, chest pain, confusion, constipation, cough, decreased CrCl, depression, dizziness, dry mouth, dry skin, dyspepsia, dyspnea, edema, eructation, headache, hypertension, increased ALT and AST, increased creatinine, insomnia, leg cramps, malaise, myalgia, myasthenia, pancytopenia, seizures, somnolence, stomatitis, taste perversion, tinnitus, tremor, and weight loss have occurred. Gastrointestinal perforation, multiple organ failure, pancreatitis, and sepsis have occurred and may be fatal. Numerous additional side effects may occur.
Overdose:
Acute renal failure, hematuria, hepatitis, irreversible pancytopenia, persistent bone marrow suppression, and seizures have occurred.
Antidote
Notify physician of all side effects; most will be treated symptomatically. Filgrastim (Neupogen, Zarxio) 1 to 10 mcg/kg/day has been used to maintain the neutrophil count. Discontinue drug if neutrophils fall below 500 cells/mm3 or platelets fall below 25,000 cells/mm3. Hydration and hemodialysis (up to 50% removal) are useful in overdose. Treat anaphylaxis and resuscitate as necessary.
Gemcitabine hydrochloride
(jem-SIGHT-ah-been hy-droh-KLOR-eyed)
Gemzar
Antineoplastic (miscellaneous)
pH 2.7 to 3.3
Usual dose
Pancreatic cancer:
1,000 mg/M2 as outlined in the following treatment schedule:
Weeks 1-8:
Weekly dosing for the first 7 weeks followed by 1 week of rest.
After week 8:
Weekly dosing on Days 1, 8, and 15 of 28-day cycles.
Non–small-cell lung cancer (NSCLC):
1,000 mg/M2 as an infusion on Days 1, 8, and 15 of each 28-day cycle. Given in combination with cisplatin. Administer cisplatin 100 mg/M2 IV on Day 1 after the infusion of gemcitabine. An alternate schedule is gemcitabine 1,250 mg/M2 on Days 1 and 8 of each 21-day cycle. Administer cisplatin 100 mg/M2 IV on Day 1 after the infusion of gemcitabine. See cisplatin monograph for administration and hydration guidelines.
Breast cancer:
1,250 mg/M2 as an infusion on Days 1 and 8 of each 21-day cycle. Given in combination with paclitaxel (Taxol). On Day 1, administer paclitaxel 175 mg/M2 as a 3-hour infusion before the gemcitabine infusion. Premedication required; see paclitaxel monograph.
Ovarian cancer:
1,000 mg/M2 as an infusion on Days 1 and 8 of a 21-day cycle. Given in combination with carboplatin (Paraplatin). On Day 1, administer carboplatin at AUC 4 after gemcitabine administration.
Bladder cancer (unlabeled):
1,000 mg/M2 on Days 1, 8, and 15. Repeat cycle every 28 days. Given in conjunction with cisplatin.
Hodgkin’s lymphoma (relapsed) or non-Hodgkin’s lymphoma (refractory) (unlabeled):
1,000 mg/M2 on Days 1 and 8 of a 21-day cycle.
Adenocarcinoma of the pancreas:
Given in combination with Abraxane; see paclitaxel protein-bound particles for injectable suspension (Abraxane) monograph.
Dose adjustments
Clearance decreased in women and the elderly. May be less likely to progress to subsequent cycles; see Precautions and Elderly.
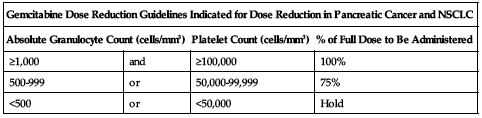
Treatment of pancreatic cancer and NSCLC:
Reduce dose based on the degree of hematologic toxicity according to the following chart.
| Gemcitabine Dose Reduction Guidelines Indicated for Dose Reduction in Pancreatic Cancer and NSCLC | |||
| Absolute Granulocyte Count (cells/mm3) | Platelet Count (cells/mm3) | % of Full Dose to Be Administered | |
| ≥1,000 | and | ≥100,000 | 100% |
| 500-999 | or | 50,000-99,999 | 75% |
| <500 | or | <50,000 | Hold |
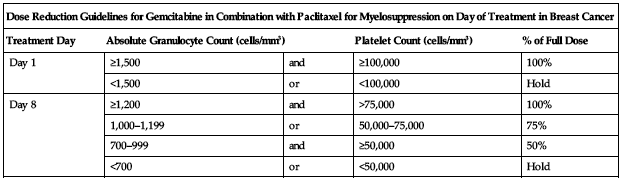
Treatment of breast cancer (combination with paclitaxel):
Reduce dose based on degree of hematologic toxicity according to the following chart.
| Dose Reduction Guidelines for Gemcitabine in Combination with Paclitaxel for Myelosuppression on Day of Treatment in Breast Cancer | ||||
| Treatment Day | Absolute Granulocyte Count (cells/mm3) | Platelet Count (cells/mm3) | % of Full Dose | |
| Day 1 | ≥1,500 | and | ≥100,000 | 100% |
| <1,500 | or | <100,000 | Hold | |
| Day 8 | ≥1,200 | and | >75,000 | 100% |
| 1,000–1,199 | or | 50,000–75,000 | 75% | |
| 700–999 | and | ≥50,000 | 50% | |
| <700 | or | <50,000 | Hold | |
See paclitaxel monograph for additional dose adjustment guidelines.
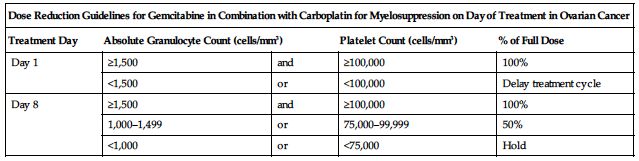
Treatment of ovarian cancer in combination with carboplatin:
Reduce dose based on the degree of hematologic toxicity according to the following charts.
| Dose Reduction Guidelines for Gemcitabine in Combination with Carboplatin for Myelosuppression on Day of Treatment in Ovarian Cancer | ||||
| Treatment Day | Absolute Granulocyte Count (cells/mm3) | Platelet Count (cells/mm3) | % of Full Dose | |
| Day 1 | ≥1,500 | and | ≥100,000 | 100% |
| <1,500 | or | <100,000 | Delay treatment cycle | |
| Day 8 | ≥1,500 | and | ≥100,000 | 100% |
| 1,000–1,499 | or | 75,000–99,999 | 50% | |
| <1,000 | or | <75,000 | Hold | |
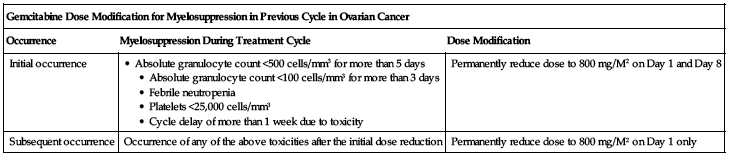
Gemcitabine dose modification for myelosuppression in previous cycle in ovarian cancer:
Reduce dose based on myelosuppression occurrence according to the chart on the following page.
| Gemcitabine Dose Modification for Myelosuppression in Previous Cycle in Ovarian Cancer | ||
| Occurrence | Myelosuppression During Treatment Cycle | Dose Modification |
| Initial occurrence | • Absolute granulocyte count <500 cells/mm3 for more than 5 days • Absolute granulocyte count <100 cells/mm3 for more than 3 days • Febrile neutropenia • Platelets <25,000 cells/mm3 • Cycle delay of more than 1 week due to toxicity | Permanently reduce dose to 800 mg/M2 on Day 1 and Day 8 |
| Subsequent occurrence | Occurrence of any of the above toxicities after the initial dose reduction | Permanently reduce dose to 800 mg/M2 on Day 1 only |
See carboplatin monograph for additional dosing guidelines.
Dilution
Specific techniques required; see precautions.
Each 200 mg must be reconstituted with 5 mL NS without preservatives (25 mL NS for 1 Gm). Yields 38 mg/mL. Shake to dissolve. Do not use less solution to reconstitute; dissolution will be incomplete. The appropriate dose must be further diluted with NS to concentrations as low as 0.1 mg/mL. 1,500 mg diluted in 250 mL yields 6 mg/mL. 750 mg in 100 mL yields 7.5 mg/mL.
Filters:
Reconstituted solution may be filtered through a 0.22- or 0.45-micron pre-filter.
Storage:
Store unopened vials at CRT. Reconstituted or diluted solutions are stable at CRT for 24 hours. Do not refrigerate in any form; may crystallize. Discard unused portion.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Manufacturer states, “Compatibilities with other drugs have not been studied. No incompatibilities observed with IV bottles or PVC bags and administration sets.”
One source suggests the following compatibilities:
Y-site:
Amifostine (Ethyol), amikacin, aminophylline, ampicillin, ampicillin/sulbactam (Unasyn), anidulafungin (Eraxis), aztreonam (Azactam), bleomycin (Blenoxane), bumetanide, buprenorphine (Buprenex), butorphanol (Stadol), calcium gluconate, carboplatin (Paraplatin), carmustine (BiCNU), cefazolin (Ancef), cefotetan (Cefotan), cefoxitin (Mefoxin), ceftazidime (Fortaz), ceftriaxone (Rocephin), cefuroxime (Zinacef), chlorpromazine (Thorazine), ciprofloxacin (Cipro IV), cisplatin, clindamycin (Cleocin), cyclophosphamide (Cytoxan), cytarabine (ARA-C), dactinomycin (Cosmegen), daunorubicin (Cerubidine), dexamethasone (Decadron), dexrazoxane (Zinecard), diphenhydramine (Benadryl), dobutamine, docetaxel (Taxotere), dopamine, doripenem (Doribax), doxorubicin (Adriamycin), doxycycline, droperidol (Inapsine), enalaprilat (Vasotec IV), etoposide (VePesid), etoposide phosphate (Etopophos), famotidine (Pepcid IV), fluconazole (Diflucan), fludarabine (Fludara), fluorouracil (5-FU), gentamicin, granisetron (Kytril), heparin, hydrocortisone sodium succinate (Solu-Cortef), hydromorphone (Dilaudid), idarubicin (Idamycin), ifosfamide (Ifex), leucovorin calcium, linezolid (Zyvox), lorazepam (Ativan), mannitol, meperidine (Demerol), mesna (Mesnex), metoclopramide (Reglan), metronidazole (Flagyl IV), mitoxantrone (Novantrone), morphine, nalbuphine, ondansetron (Zofran), oxaliplatin (Eloxatin), paclitaxel (Taxol), palonosetron (Aloxi), potassium chloride (KCl), promethazine (Phenergan), ranitidine (Zantac), sodium bicarbonate, streptozocin (Zanosar), sulfamethoxazole/trimethoprim, teniposide (Vumon), thiotepa, ticarcillin/clavulanate (Timentin), tobramycin, topotecan (Hycamtin), vancomycin, vinblastine, vincristine, vinorelbine (Navelbine), zidovudine (AZT, Retrovir).
Rate of administration
A single dose as an infusion equally distributed over 30 minutes. Do not extend infusion time beyond 60 minutes; will increase toxicity.
Actions
A nucleoside metabolic inhibitor with antineoplastic activity. Metabolized intracellularly to two active nucleosides. Cell phase specific, these nucleosides induce internucleosomal DNA fragmentation, primarily killing cells undergoing DNA synthesis (S-phase) and also blocking the progression of cells through the G1/S-phase boundary. Very little is bound to plasma protein. Volume of distribution is increased by infusion length. Half-life is shorter (42 to 94 minutes) with a short infusion (less than 70 minutes), and longer (245 to 638 minutes) with a long infusion (more than 70 minutes). Half-life is slightly longer and rate of clearance is lower in women and in the elderly, resulting in higher concentrations for any given dose. Primarily excreted in urine.
Indications and uses
First-line treatment for patients with locally advanced (nonresectable Stage II or Stage III) or metastatic (Stage IV) adenocarcinoma of the pancreas in patients previously treated with fluorouracil. ■ First-line treatment in combination with cisplatin for the treatment of inoperable, locally advanced (Stage IIIA or IIIB) or metastatic (Stage IV) non–small-cell lung cancer. ■ First-line treatment in combination with paclitaxil for treatment of metastatic breast cancer after failure of previous anthracycline chemotherapy unless anthracyclines (e.g., doxorubicin [Adriamycin], idarubicin [Idamycin]) were clinically contraindicated. ■ Treatment in combination with carboplatin for patients with advanced ovarian cancer that has relapsed at least 6 months after completion of platinum-based therapy.
Unlabeled uses:
Treatment of metastatic bladder cancer. ■ Treatment of testicular cancer. ■ Treatment of cancer of the head and neck.
Contraindications
Hypersensitivity to gemcitabine or any of its components.
Precautions
Follow guidelines for handling cytotoxic agents. See Appendix A, p. 1331. ■ Administered by or under the direction of the physician specialist. ■ Adequate diagnostic and treatment facilities must be available. ■ For IV use only. May be administered on an outpatient basis. ■ Prolongation of the infusion time beyond 60 minutes and more frequent than weekly dosing have been shown to increase toxicity (e.g., clinically significant hypotension, severe flu-like symptoms, myelosuppression, and asthenia). ■ Myelosuppression (e.g., anemia, neutropenia, and thrombocytopenia) is the dose-limiting toxicity. Occurs with gemcitabine as a single agent, and the risk increases when it is combined with other cytotoxic drugs; see Dose Adjustments. ■ Clearance in women and the elderly is reduced; women, especially older women, were more likely not to proceed to a subsequent cycle and to experience Grade 3 or 4 neutropenia and thrombocytopenia. No age or gender dose adjustments recommended. ■ Use with caution in impaired renal or hepatic function. Clear dose recommendations are not available; data from clinical studies insufficient. ■ Hepatotoxicity, including liver failure and death, has been reported. Use in patients with concurrent liver metastases or a history of alcoholism, hepatitis, or liver cirrhosis may lead to exacerbation of the underlying hepatic insufficiency. ■ Hemolytic-uremic syndrome (HUS) and/or renal toxicity, including renal failure leading to death or requiring dialysis, has been reported. ■ Gemcitabine is a potent radiosensitizer. Depending on the site being radiated, concurrent use with gemcitabine may cause severe, life-threatening esophagitis and pneumonitis. Data suggest that gemcitabine can be started after the acute effects of radiation have resolved or at least 1 week after radiation is completed. Radiation recall has been reported in patients who receive gemcitabine after prior radiation. ■ Pulmonary toxicity (e.g., adult respiratory distress syndrome [ARDS], interstitial pneumonitis, pulmonary edema, pulmonary fibrosis) has been reported. Fatalities have occurred. Onset of pulmonary symptoms may occur up to 2 weeks after the last dose of gemcitabine. Discontinue gemcitabine in patients who develop unexplained dyspnea, with or without bronchospasm, or who have any evidence of pulmonary toxicity. ■ Capillary leak syndrome (CLS) and posterior reversible encephalopathy syndrome (PRES) have been reported. ■ Use caution in patients who have had previous cytotoxic chemotherapy or radiation therapy.
Monitor:
Monitor for bone marrow suppression. ■ Obtain a CBC, including differential and platelet count, before each dose; see Dose Adjustments. ■ Obtain baseline renal function (e.g., SCr) and liver function tests (e.g., AST, ALT) and repeat periodically. ■ Monitor serum calcium, magnesium, potassium, and SCr during combination therapy with cisplatin. ■ Monitor vital signs. ■ Maintain adequate hydration. ■ Nausea and vomiting are frequent and were severe in 15% of patients; prophylactic administration of antiemetics will increase patient comfort. ■ Observe closely for S/S of infection. May cause fever in the absence of infection, or prophylactic antibiotics may be indicated pending results of C/S in a febrile or nonfebrile patient. ■ Monitor for thrombocytopenia (platelet count less than 50,000/mm3). Initiate precautions to prevent excessive bleeding (e.g., inspect IV sites, skin, and mucous membranes; use extreme care during invasive procedures; test urine, emesis, stool, and secretions for occult blood). ■ Consider a diagnosis of HUS if anemia with evidence of microangiopathic hemolysis, elevation of bilirubin or LDH, reticulocytosis, severe thrombocytopenia, and/or evidence of renal failure (elevation of SCr or BUN) develops; discontinue gemcitabine. ■ Monitor for S/S of CLS (sudden edema, rapid drop in blood pressure, shock, hemoconcentration, hypoalbuminemia). ■ Monitor for S/S of PRES (blindness, confusion, headache, hypertension, lethargy, seizure, or other visual or neurologic disturbances). Confirm diagnosis of PRES with MRI. ■ Not a vesicant, but monitor injection site for inflammation and/or extravasation.
Patient education:
Nonhormonal birth control recommended. ■ See Appendix D, p. 1333. ■ Report any unusual or unexpected symptoms or side effects (e.g., shortness of breath, cough, blood in stool or urine, change in color or volume of urine, S/S of infection, jaundice, unusual bruising or bleeding) as soon as possible.
Maternal/child:
Category D: avoid pregnancy. May cause fetal harm. ■ Discontinue breast-feeding. ■ Safety and effectiveness for use in pediatric patients not established.
Elderly:
Clearance reduced in the elderly; hematologic toxicity requiring reduction, delay, or omission of subsequent doses is higher than in younger adults; however, incidence of nonhematologic toxicity is similar. Elderly men and women are more likely to experience Grade 3 or 4 thrombocytopenia. Elderly women are also more likely to experience Grade 3 or 4 neutropenia. Usual dose adjustments based on toxicity are considered appropriate. Age-related impaired renal function may further reduce clearance and increase toxicity.
Drug/lab interactions
Interaction of gemcitabine with other drugs has not been adequately studied. ■ Additive bone marrow suppression may occur with radiation therapy, other bone marrow–suppressing agents (e.g., amphotericin B [traditional and lipid], azathioprine, chloramphenicol, melphelan [Alkeran]), and/or immunosuppressants (e.g., chlorambucil [Leukeran], cyclophosphamide [Cytoxan], cyclosporine [Sandimmune], glucocorticoid corticosteroids [e.g., dexamethasone], muromonab-CD3 [Orthoclone], tacrolimus [Prograf]); dose reduction may be required. ■ Do not administer live virus vaccines to patients receiving antineoplastic agents.
Side effects
The most common side effects are anemia, dyspnea, fever, hematuria, hepatic transaminitis, increased alkaline phosphatase, nausea, neutropenia, peripheral edema, proteinuria, rash, thrombocytopenia, and vomiting. Less frequently reported side effects include alopecia, anorexia, arrhythmias, arthralgia, bone marrow toxicity (e.g., anemia, leukopenia, neutropenia, thrombocytopenia), bone pain, bronchospasm, capillary leak syndrome, cerebrovascular accident, CHF, constipation, diarrhea, edema, elevated lab tests (e.g., BUN, creatinine, hematuria, proteinuria), fatigue, febrile neutropenia, flu syndrome (e.g., anorexia, chills, cough, headache, myalgia, weakness), hemolytic-uremic syndrome, hemorrhage, hepatotoxicity, hypertension, increased liver function tests (e.g., ALT, AST, GGT, alkaline phosphatase, and bilirubin), infection, injection site reaction, myalgia, myocardial infarction, neuropathy (motor and sensory), pain, paresthesias, peripheral vasculitis and gangrene, posterior reversible encephalopathy syndrome (PRES), pruritus, pulmonary toxicity (including pulmonary edema, pulmonary fibrosis, interstitial pneumonitis, ARDS, respiratory failure), radiation recall reactions, severe skin reactions (e.g., desquamation and bullous skin eruptions), somnolence, and stomatitis. Anaphylaxis and hemolytic-uremic syndrome have been reported.
Antidote
Keep physician informed of all side effects. Symptomatic and supportive treatment is indicated. Permanently discontinue gemcitabine if any of the following occur: unexplained dyspnea or other evidence of severe pulmonary toxicity, severe hepatic toxicity, hemolytic-uremic syndrome, capillary leak syndrome, or posterior reversible encephalopathy syndrome (PRES). Reduce dose or withhold gemcitabine until myelosuppression improves to specific criteria; see Dose Adjustments. If gemcitabine-induced pneumonitis or esophagitis is confirmed or suspected, discontinue permanently (in one study severe stomatitis and pharyngeal damage required patients to be fed by feeding tube for up to 12 months after receiving doses of 300 mg/M2 [25% of the usual dose]). Anemia may require RBC transfusions. Other whole blood products (e.g., platelets, leukocytes) and/or blood modifiers (e.g., darbepoetin alfa [Aranesp], epoetin alfa [Epogen], filgrastim [Neupogen, Zarxio], pegfilgrastim [Neulasta], sargramostim [Leukine]) may be indicated to treat bone marrow toxicity. Most side effects are reversible with dose reduction or temporary withholding of gemcitabine. No known antidote for overdose. If hemolytic-uremic syndrome occurs, discontinue gemcitabine; renal failure may not be reversible even with discontinuation of therapy, and dialysis may be required. Treat hypersensitivity reactions as indicated; may require epinephrine, airway management, oxygen, IV fluids, antihistamines (e.g., diphenhydramine [Benadryl]), corticosteroids (e.g., hydrocortisone sodium succinate [Solu-Cortef]), and pressor amines (e.g., dopamine).
Gentamicin sulfate 
(jen-tah-MY-sin SUL-fayt)
Antibacterial (aminoglycoside)
pH 3 to 5.5
Usual dose
3 mg/kg of body weight/24 hr equally divided into 3 doses (1 mg/kg every 8 hours). Up to 5 mg/kg/24 hr may be given if indicated. Reduce to usual dose as soon as feasible. Another source suggests 1 to 2.5 mg/kg/dose every 8 to 12 hours. A loading dose of 2 mg/kg is commonly used. Dosage based on ideal body weight. Studies suggest that in certain populations a single daily dose of 4 to 7 mg/kg (instead of divided into 2 to 3 doses) may provide higher peak levels and enhance drug effectiveness while actually reducing or having no adverse effects on risk of toxicity. Various procedures for monitoring blood levels are in use. Some health facilities are monitoring with trough levels; others may draw levels at predetermined times and plot the concentration on nomograms. Depending on the protocol in place, doses or intervals may be adjusted. See Dose Adjustments and Precautions/Monitor.
Prevention of bacterial endocarditis in dental, respiratory tract, GI or GU tract surgery or instrumentation:
1.5 mg/kg 30 minutes before procedure. Do not exceed 80 mg. Repeat in 8 hours. Given concurrently with ampicillin, vancomycin, or amoxicillin.
Pelvic inflammatory disease:
2 mg/kg as an initial dose. Follow with 1.5 mg/kg every 8 hours for 4 days or 48 hours after patient improves. Given concurrently with clindamycin.
Pediatric dose
See Maternal/Child.
6 to 7.5 mg/kg of body weight/24 hr (2 to 2.5 mg/kg every 8 hours). A single daily dose is also being used in pediatric patients. See comments under Usual Dose. 10 mg/mL product available for pediatric use.
Prevention of bacterial endocarditis in dental, respiratory tract, GI or GU tract surgery or instrumentation:
2 mg/kg. See Adult Dose for instructions.
Neonatal dose
See Maternal/Child.
2.5 mg/kg. Intervals adjusted based on age as follows:
0 to 7 days of age; less than 28 weeks’ gestation: Every 24 hours.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


