Fluid, Electrolyte, and Acid-Base Balance
Objectives
• Describe the processes involved in regulating acid-base balance.
• Describe common fluid, electrolyte, and acid-base imbalances.
• Identify risk factors for fluid, electrolyte, and acid-base imbalances.
• Choose appropriate clinical assessments for specific fluid, electrolyte, and acid-base imbalances.
• Interpret basic fluid, electrolyte, and acid-base laboratory values.
• Discuss purpose and procedure for initiation and maintenance of intravenous therapy.
• Calculate an intravenous flow rate.
• Describe how to measure and record fluid intake and output.
• Explain how to change intravenous solutions and tubing and discontinue an infusion.
• Describe potential complications of intravenous therapy and what to do if they occur.
Key Terms
Acidosis, p. 893
Active transport, p. 884
Alkalosis, p. 893
Anion gap, p. 893
Anions, p. 883
Arterial blood gases (ABGs), p. 891
Autologous transfusion, p. 911
Buffers, p. 891
Cations, p. 883
Colloid osmotic pressure, p. 885
Colloids, p. 885
Crystalloids, p. 904
Dehydration, p. 887
Electrolytes, p. 883
Electronic infusion devices (EIDs), p. 907
Extracellular fluid (ECF), p. 883
Extracellular fluid volume deficit (ECV deficit), p. 887
Extracellular fluid volume excess (ECV excess), p. 887
Extravasation, p. 909
Filtration, p. 884
Fluid, p. 883
Hydrostatic pressure, p. 885
Hypercalcemia, p. 890
Hyperkalemia, p. 889
Hypermagnesemia, p. 891
Hypernatremia, p. 887
Hypertonic, p. 884
Hypocalcemia, p. 889
Hypokalemia, p. 889
Hypomagnesemia, p. 890
Hyponatremia, p. 887
Hypotonic, p. 884
Hypovolemia, p. 887
Infiltration, p. 909
Interstitial fluid, p. 883
Intracellular fluid (ICF), p. 883
Intravascular fluid, p. 883
Ions, p. 883
Isotonic, p. 884
Metabolic acidosis, p. 893
Metabolic alkalosis, p. 893
Oncotic pressure, p. 885
Osmolality, p. 883
Osmosis, p. 884
Osmotic pressure, p. 884
Phlebitis, p. 909
Respiratory acidosis, p. 893
Respiratory alkalosis, p. 893
Transcellular fluids, p. 883
Transfusion reaction, p. 909
Vascular access devices (VADs), p. 906
Venipuncture, p. 906
![]()
• Skills Performance Checklists
• Interactive Learning Activities
• Butterfield’s Fluids and Electrolytes Tutorial
Fluid surrounds all the cells in the body and is also inside cells. Body fluids contain electrolytes such as sodium and potassium; they also have a certain degree of acidity. Fluid, electrolyte, and acid-base balances within the body maintain the health and function of all body systems. The characteristics of body fluids influence body system function because of their effects on cell function. These characteristics include the fluid amount (volume), concentration (osmolality), composition (electrolyte concentration), and degree of acidity (pH). All of these characteristics have regulatory mechanisms, which keeps them in balance for normal function. In this chapter you learn how the body normally maintains fluid, electrolyte, and acid-base balance. You also learn how imbalances develop; how various fluid, electrolyte, and acid-base imbalances affect patients; and ways to help patients maintain or restore balance safely.
Scientific Knowledge Base
This section provides the foundation for your critical thinking regarding patients who have or are at risk of having, fluid, electrolyte, or acid-base imbalances.
Location and Movement of Water and Electrolytes
Water makes up a substantial proportion of body weight. In fact, about 60% of the body weight of an adult man is water. This proportion decreases with age; approximately 50% of an older man’s weight is water. Women typically have less water content than men. Obese people have less water in their bodies than lean people because fat contains less water than muscle. The term fluid means water that contains dissolved or suspended substances such as glucose, mineral salts, and proteins.
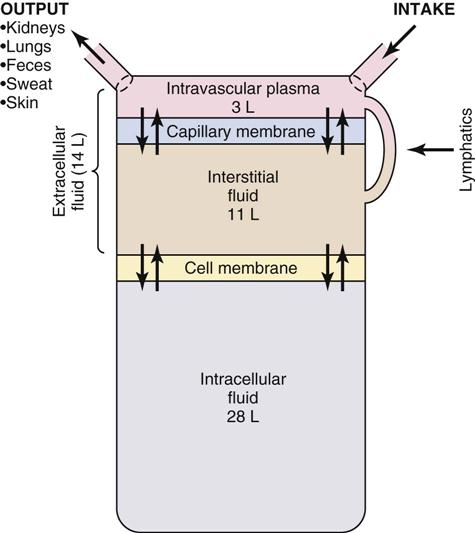
Fluid Compartments.
Body fluids are located in two distinct compartments: extracellular fluid (ECF) outside the cells, and intracellular fluid (ICF) inside the cells (Fig. 41-1). In adults ICF is approximately two thirds of total body water. ECF is approximately one third of total body water. ECF has two major divisions (intravascular fluid and interstitial fluid) and a minor division (transcellular fluids). Intravascular fluid is the liquid portion of the blood (i.e., the plasma). Interstitial fluid is located between the cells and outside the blood vessels. Transcellular fluids such as cerebrospinal, pleural, peritoneal, and synovial fluids are secreted by epithelial cells (Hall, 2011).
Fluid in the body compartments contains mineral salts known technically as electrolytes. An electrolyte is a compound that separates into ions (charged particles) when it dissolves in water. Ions that are positively charged are called cations; ions that are negatively charged are called anions. Cations in body fluids are sodium (Na+), potassium (K+), calcium (Ca2+), and magnesium ions (Mg2+). Anions in body fluids are chloride (Cl−) and bicarbonate (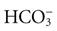 ). Anions and cations combine to make salts. If you put table salt (NaCl) in water, it separates into Na+ and Cl−. Other combinations of anions and cations do the same. Clinical laboratories usually report electrolyte measurements in milliequivalents per liter (mEq/L) or millimoles per liter (mmol/L), two different units of concentration (Table 41-1). Millimoles per liter represent the number of milligrams of the electrolyte divided by its molecular weight that are contained in a liter of the fluid being measured (usually blood plasma or serum). Milliequivalents per liter is the millimoles per liter multiplied by the electrolyte charge (e.g., 1 for Na+, 2 for Ca2+). A milliequivalent of one electrolyte can combine with a milliequivalent of another electrolyte, which is why this measurement unit is used (Rose, 2011).
). Anions and cations combine to make salts. If you put table salt (NaCl) in water, it separates into Na+ and Cl−. Other combinations of anions and cations do the same. Clinical laboratories usually report electrolyte measurements in milliequivalents per liter (mEq/L) or millimoles per liter (mmol/L), two different units of concentration (Table 41-1). Millimoles per liter represent the number of milligrams of the electrolyte divided by its molecular weight that are contained in a liter of the fluid being measured (usually blood plasma or serum). Milliequivalents per liter is the millimoles per liter multiplied by the electrolyte charge (e.g., 1 for Na+, 2 for Ca2+). A milliequivalent of one electrolyte can combine with a milliequivalent of another electrolyte, which is why this measurement unit is used (Rose, 2011).
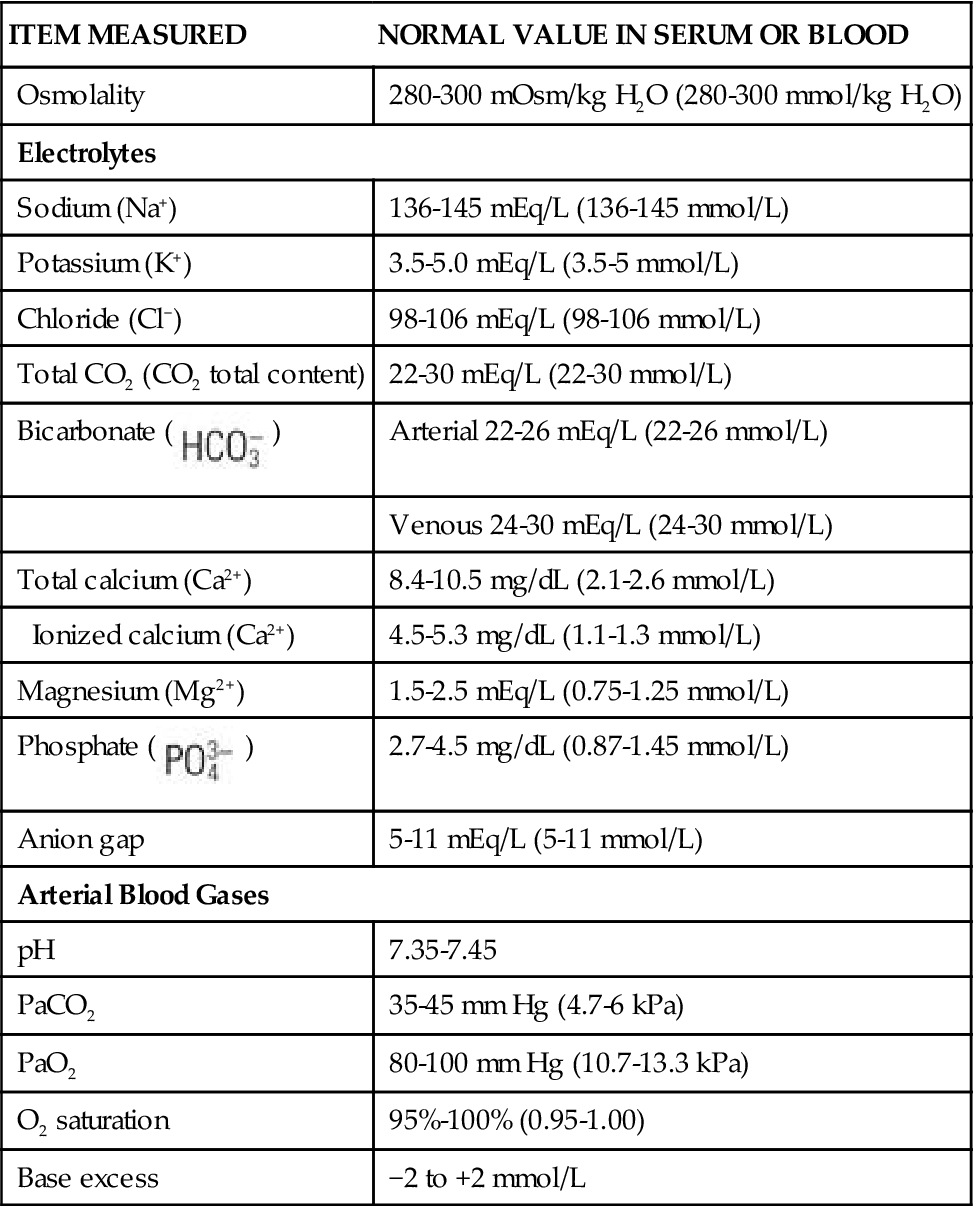
TABLE 41-1
Laboratory Normal Values for Adults
| ITEM MEASURED | NORMAL VALUE IN SERUM OR BLOOD |
| Osmolality | 280-300 mOsm/kg H2O (280-300 mmol/kg H2O) |
| Electrolytes | |
| Sodium (Na+) | 136-145 mEq/L (136-145 mmol/L) |
| Potassium (K+) | 3.5-5.0 mEq/L (3.5-5 mmol/L) |
| Chloride (Cl−) | 98-106 mEq/L (98-106 mmol/L) |
| Total CO2 (CO2 total content) | 22-30 mEq/L (22-30 mmol/L) |
Bicarbonate (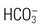 ) ) | Arterial 22-26 mEq/L (22-26 mmol/L) |
| Venous 24-30 mEq/L (24-30 mmol/L) | |
| Total calcium (Ca2+) | 8.4-10.5 mg/dL (2.1-2.6 mmol/L) |
| Ionized calcium (Ca2+) | 4.5-5.3 mg/dL (1.1-1.3 mmol/L) |
| Magnesium (Mg2+) | 1.5-2.5 mEq/L (0.75-1.25 mmol/L) |
Phosphate (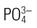 ) ) | 2.7-4.5 mg/dL (0.87-1.45 mmol/L) |
| Anion gap | 5-11 mEq/L (5-11 mmol/L) |
| Arterial Blood Gases | |
| pH | 7.35-7.45 |
| PaCO2 | 35-45 mm Hg (4.7-6 kPa) |
| PaO2 | 80-100 mm Hg (10.7-13.3 kPa) |
| O2 saturation | 95%-100% (0.95-1.00) |
| Base excess | −2 to +2 mmol/L |
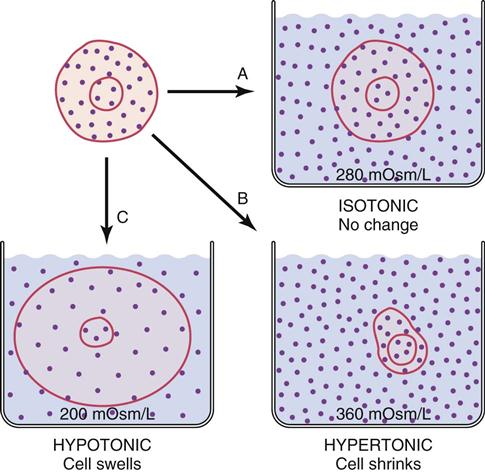
Fluid that contains a large number of dissolved particles is more concentrated than the same amount of fluid that contains only a few particles. Osmolality of a fluid is a measure of the number of particles per kilogram of water. Some particles (e.g., urea) pass easily through cell membranes; others such as Na+ cannot cross easily. The particles that cannot cross cell membranes easily (nonpermeant particles) determine tonicity of a fluid (Caon, 2008). A fluid with the same concentration of nonpermeant particles as normal blood is called isotonic. A hypotonic solution is more dilute than the blood, and a hypertonic solution is more concentrated than normal blood (Fig. 41-2).
Movement of Water and Electrolytes.
Active transport, diffusion, osmosis, and filtration are processes that move water and electrolytes between body compartments. These processes maintain equal osmolality in all compartments while allowing for different electrolyte concentrations.
Active Transport.
Fluids in different body compartments have different concentrations of electrolytes that are necessary for normal function. For example, concentrations of Na+, Cl−, and 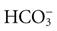 are higher in the ECF than in the ICF, whereas the concentrations of K+, Mg2+, and PO3−4 are higher in the ICF than in the ECF. Cells maintain their high intracellular electrolyte concentration by active transport. Active transport requires energy in the form of adenosine triphosphate (ATP) to move electrolytes across cell membranes against the concentration gradient (from areas of lower concentration to areas of higher concentration). One example of active transport is the sodium-potassium pump, which moves Na+ out of a cell and K+ into it, keeping ICF lower in Na+ and higher in K+ than the ECF.
are higher in the ECF than in the ICF, whereas the concentrations of K+, Mg2+, and PO3−4 are higher in the ICF than in the ECF. Cells maintain their high intracellular electrolyte concentration by active transport. Active transport requires energy in the form of adenosine triphosphate (ATP) to move electrolytes across cell membranes against the concentration gradient (from areas of lower concentration to areas of higher concentration). One example of active transport is the sodium-potassium pump, which moves Na+ out of a cell and K+ into it, keeping ICF lower in Na+ and higher in K+ than the ECF.
Diffusion.
Diffusion is passive movement of electrolytes or other particles down the concentration gradient (from areas of higher concentration to areas of lower concentration). Within a body compartment electrolytes diffuse easily by random movements until the concentration is the same in all areas. However, diffusion of electrolytes across cell membranes requires proteins that serve as ion channels. For example, when a sodium channel in a cell membrane is open, Na+ diffuses passively across the cell membrane into the ICF because concentration is lower in the ICF. Opening of ion channels is tightly controlled and plays an important part in muscle and nerve function.
Osmosis.
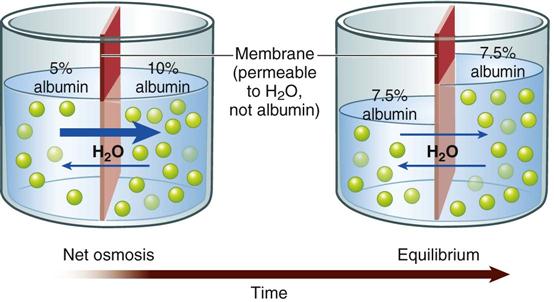
Water moves across cell membranes by osmosis, a process by which water moves through a membrane that separates fluids with different particle concentrations (Fig. 41-3). Cell membranes are semipermeable, which means that water crosses them easily but they are not freely permeable to many types of particles, including electrolytes such as sodium and potassium. These semipermeable cell membranes separate interstitial fluid from ICF. The fluid in each of these compartments exerts osmotic pressure, an inward-pulling force caused by particles in the fluid. The particles already inside the cell exert ICF osmotic pressure, which tends to pull water into the cell. The particles in the interstitial fluid exert interstitial fluid osmotic pressure, which tends to pull water out of the cell. Water moves into the compartment that has a higher osmotic pressure (inward-pulling force) until the particle concentration is equal in the two compartments.
If the particle concentration in the interstitial compartment changes, osmosis occurs rapidly and moves water into or out of cells to equalize the osmotic pressures. For example, when a hypotonic solution (more dilute than normal body fluids) is administered intravenously, it dilutes the interstitial fluid, decreasing its osmotic pressure below intracellular osmotic pressure. Water moves rapidly into cells until the two osmotic pressures are equal again. On the other hand, infusion of a hypertonic intravenous (IV) solution (more concentrated than normal body fluids) causes water to leave cells by osmosis to equalize the osmolality between interstitial and intracellular compartments.
Filtration.
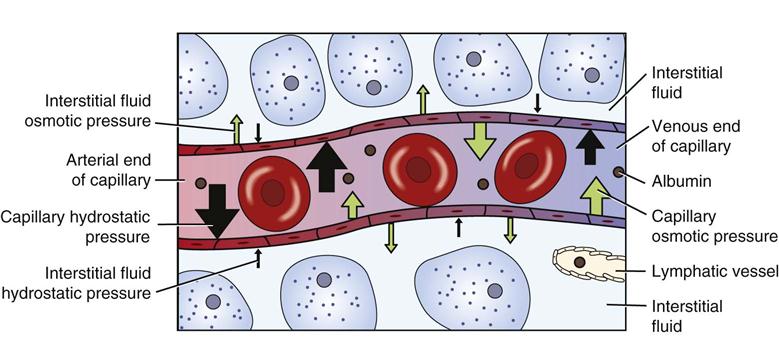
Fluid moves into and out of capillaries (between the vascular and interstitial compartments) by the process of filtration (Fig. 41-4). Filtration is the net effect of four forces, two that tend to move fluid out of capillaries and small venules and two that tend to move fluid back into them (Rose, 2011). Hydrostatic pressure is the force of the fluid pressing outward against a surface. Similarly, capillary hydrostatic pressure is a relatively strong outward-pushing force that helps move fluid from capillaries into the interstitial area. Interstitial fluid hydrostatic pressure is a weaker opposing force that tends to push fluid back into capillaries.
Blood contains albumin and other proteins known as colloids. These proteins are much larger than electrolytes, glucose, and other molecules that dissolve easily. Most colloids are too large to leave capillaries in the fluid that is filtered, so they remain in the blood. Because they are particles, colloids exert osmotic pressure. Blood colloid osmotic pressure, also called oncotic pressure, is an inward-pulling force caused by blood proteins that helps move fluid from the interstitial area back into capillaries. Interstitial fluid colloid osmotic pressure normally is a very small opposing force.
At the arterial end of a normal capillary, capillary hydrostatic pressure is strongest, and fluid moves from the capillary into the interstitial area, bringing nutrients to cells. At the venous end capillary hydrostatic pressure is weaker, and the colloid osmotic pressure of the blood is stronger. Thus fluid moves into the capillary at the venous end, removing waste products from cellular metabolism. Lymph vessels remove any extra fluid and proteins that have leaked into the interstitial fluid.
Disease processes and other factors that alter these forces may cause accumulation of excess fluid in the interstitial space, known as edema. For example, people with heart failure develop edema. In this situation, venous congestion from a weakened heart, which no longer pumps effectively, increases capillary hydrostatic pressure, causing edema by moving excessive fluid into the interstitial space. Inflammation is another cause of edema. It increases capillary blood flow and allows capillaries to leak colloids into the interstitial space. The resulting increased capillary hydrostatic pressure and increased interstitial colloid osmotic pressure produce localized edema in the inflamed tissues.
Fluid Balance
Fluid homeostasis is the dynamic interplay of three processes: fluid intake and absorption, fluid distribution, and fluid output (Felver, 2010b). Human total daily fluid output consists of hypotonic sodium-containing fluid. People must have intake of an equivalent amount of hypotonic sodium-containing fluid (or water plus foods with some salt) to maintain fluid balance.
Fluid Intake.
Fluid intake occurs orally through drinking but also through eating because most foods contain some water. Food metabolism creates additional water. Average fluid intake from these routes for healthy adults is about 2300 mL, although it varies widely (Table 41-2). Other routes of fluid intake include IV, rectal (e.g., enemas), and irrigation of body cavities that can absorb fluid.
TABLE 41-2
Healthy Adult Average Daily Fluid Intake and Output
| FLUID INTAKE | NORMAL | PROLONGED HEAVY EXERCISE |
| Fluids ingested: | ||
| Oral | 1100-1400 mL | ? |
| Foods | 800-1000 mL | ? |
| Metabolism | 300 mL | 200 mL |
| TOTAL | 2200-2700 mL | ? |
| FLUID OUTPUT | ||
| Skin (insensible and sweat) | 500-600 mL | 5350 mL |
| Insensible-lungs | 400 mL | 650 mL |
| GI | 100-200 mL | 100 mL |
| Urine | 1200-1500 mL | 500 mL |
| TOTAL | 2200-2700 mL | 6600 mL |
Data from Heitz UE, Horne MM: Mosby’s pocket guide series: Fluid, electrolyte and acid base balance, ed 5, St Louis, 2005, Mosby; Hall JE: Guyton and Hall textbook of medical physiology, ed 12, Philadelphia, 2011, Saunders.
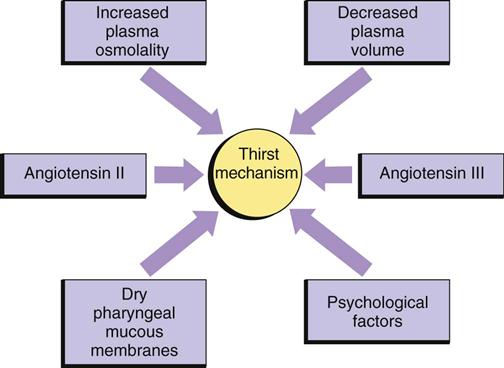
Although you might think that the major regulator of oral fluid intake is thirst, habit and social reasons actually account for most fluid intake (Johnson, 2007). Thirst, the conscious desire for water, is an important regulator of fluid intake when plasma osmolality increases (osmoreceptor-mediated thirst) or the blood volume decreases (baroreceptor-mediated thirst and angiotensin II– and III–mediated thirst). The thirst-control mechanism is located within the hypothalamus in the brain (Fig. 41-5). Osmoreceptors continually monitor plasma osmolality; when it increases, they cause thirst by stimulating neurons in the hypothalamus. Dry oral mucous membranes also cause thirst. People who are alert can obtain fluid or communicate their thirst to others, and fluid intake restores fluid balance. Infants, patients with neurological or psychological problems, and some older adults who are unable to perceive or communicate their thirst are at risk for dehydration.
Fluid Distribution.
The term fluid distribution means the movement of fluid among its various compartments. Fluid distribution between the extracellular and intracellular compartments occurs by osmosis. Fluid distribution between the vascular and interstitial portions of the ECF occurs by filtration.
Fluid Output.
Fluid output normally occurs through four organs: the skin, lungs, gastrointestinal (GI) tract, and kidneys. Examples of abnormal fluid output include vomiting, wound drainage, or hemorrhage (Felver, 2010b). Table 41-2 shows average amounts of fluid excretion for healthy adults, although urine output varies greatly, depending on fluid intake. Insensible (not visible) water loss through the skin and lungs is continuous. It increases when a person has a fever or a recent burn to the skin (Metheny, 2010). Sweat, which is visible and contains sodium, occurs intermittently and increases fluid output substantially. The GI tract plays a vital role in fluid balance. Approximately 3 to 6 L of fluid moves into the GI tract daily and then returns again to the ECF. The average adult normally excretes only 100 mL of fluid each day through feces. However, diarrhea causes a large fluid output from the GI tract.
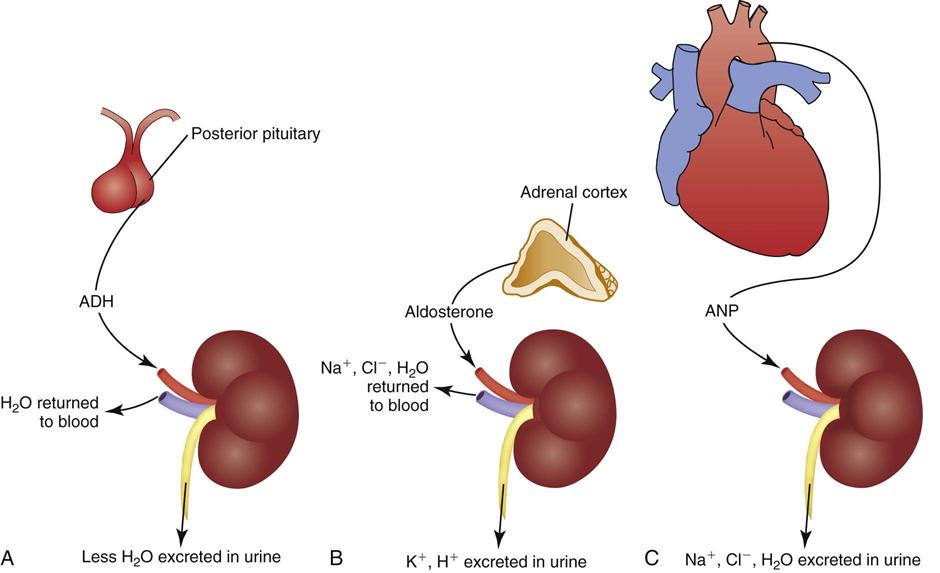
The kidneys are the major regulator of fluid output because they respond to hormones that influence urine production. When healthy adults drink more water, they increase urine production to maintain fluid balance. If they drink less water, sweat a lot, or lose fluid by vomiting, their urine volume decreases to maintain fluid balance. These adjustments primarily are caused by the actions of antidiuretic hormone (ADH), the renin-angiotensin-aldosterone system (RAAS), and atrial natriuretic peptides (ANPs) (Goldstein et al., 2010) (Fig. 41-6).
Antidiuretic Hormone.
ADH regulates the osmolality of the body fluids by influencing how much water is excreted in urine. It is synthesized by neurons in the hypothalamus that release it from the posterior pituitary gland. ADH circulates in the blood to the kidneys, where it acts on the collecting ducts (Koeppen and Stanton, 2008). Its name—antidiuretic hormone—tells you what it does. It causes renal cells to resorb water, taking water from the renal tubular fluid and putting it back in the blood. This action decreases urine volume, concentrating the urine while diluting the blood by adding water to it (see Fig. 41-6, A).
People normally have some ADH release to maintain fluid balance. More ADH is released if body fluids become more concentrated. Factors that increase ADH levels include severely decreased blood volume (e.g., dehydration, hemorrhage), pain, stressors, and some medications.
ADH levels decrease if body fluids become too dilute. This allows more water to be excreted in urine, creating a larger volume of dilute urine and concentrating the body fluids back to normal osmolality. For example, ethyl alcohol decreases ADH release, which causes people to urinate frequently when they drink alcoholic beverages.
Renin-Angiotensin-Aldosterone System.
The renin-angiotensin-aldosterone system (RAAS) regulates ECF volume by influencing how much sodium and water are excreted in urine. It also contributes to regulation of blood pressure. Specialized cells in the kidneys release the enzyme renin, which acts on angiotensinogen, an inactive protein secreted by the liver that circulates in the blood. Renin converts angiotensinogen to angiotensin I, which other enzymes in the lung capillaries convert to angiotensin II (Koeppen and Stanton, 2008). Angiotensin II has several functions, one of which is vasoconstriction in some vascular beds. The important fluid homeostasis functions of angiotensin II include stimulation of aldosterone release from the adrenal cortex.
Aldosterone circulates to the kidneys, where it causes resorption of sodium and water in isotonic proportion in the distal renal tubules. Removing sodium and water from the renal tubules and returning it to the blood increases the volume of the ECF (see Fig. 41-6, B). Aldosterone also contributes to electrolyte and acid-base balance by increasing urinary excretion of potassium and hydrogen ions.
To maintain fluid balance, normally some action of the RAAS occurs. Certain stimuli increase or decrease the activity of this system to restore fluid balance. For example, if hemorrhage or vomiting decreases the extracellular fluid volume (ECV), blood flow decreases through the renal arteries, and more renin is released. This increased RAAS activity causes more sodium and water retention, helping to restore ECV.
Atrial Natriuretic Peptide.
Atrial natriuretic peptide (ANP) also regulates ECV by influencing how much sodium and water are excreted in urine. Cells in the atria of the heart release ANP when they are stretched (e.g., by an increased ECV). ANP is a weak hormone that inhibits ADH by increasing the loss of sodium and water in the urine (see Fig. 41-6, C). Thus ANP opposes the effect of aldosterone (Koeppen and Stanton, 2008).
Fluid Imbalances
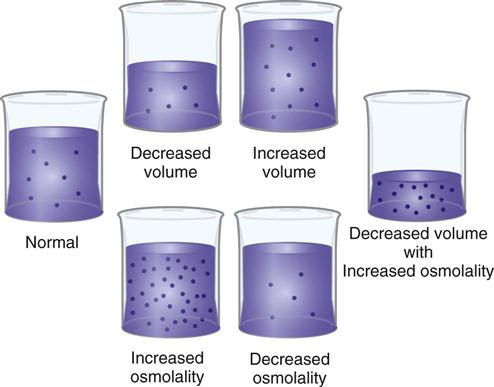
If disease processes, medications, or other factors disrupt fluid intake or output, imbalances sometimes occur (Felver, 2010b). For example, with diarrhea there is an increase in fluid output, and a fluid imbalance (dehydration) occurs if fluid intake does not increase appropriately. There are two major types of fluid imbalances: volume imbalances and osmolality imbalances (Fig. 41-7). Volume imbalances are disturbances of the amount of fluid in the extracellular compartment. Osmolality imbalances are disturbances of the concentration of body fluids. Volume and osmolality imbalances occur separately or in combination.
Extracellular Fluid Volume Imbalances.
In an ECV imbalance there is either too little (ECV deficit) or too much (ECV excess) isotonic fluid. ECV deficit is present when there is insufficient isotonic fluid in the extracellular compartment. Remember that there is a lot of sodium in normal ECF. With ECV deficit, output of isotonic fluid exceeds intake of sodium-containing fluid. Because ECF is both vascular and interstitial, signs and symptoms arise from lack of volume in both of these compartments. Table 41-3 lists specific causes and signs and symptoms of ECV deficit. The term hypovolemia means decreased vascular volume and often is used when discussing ECV deficit (Metheny, 2010).
TABLE 41-3
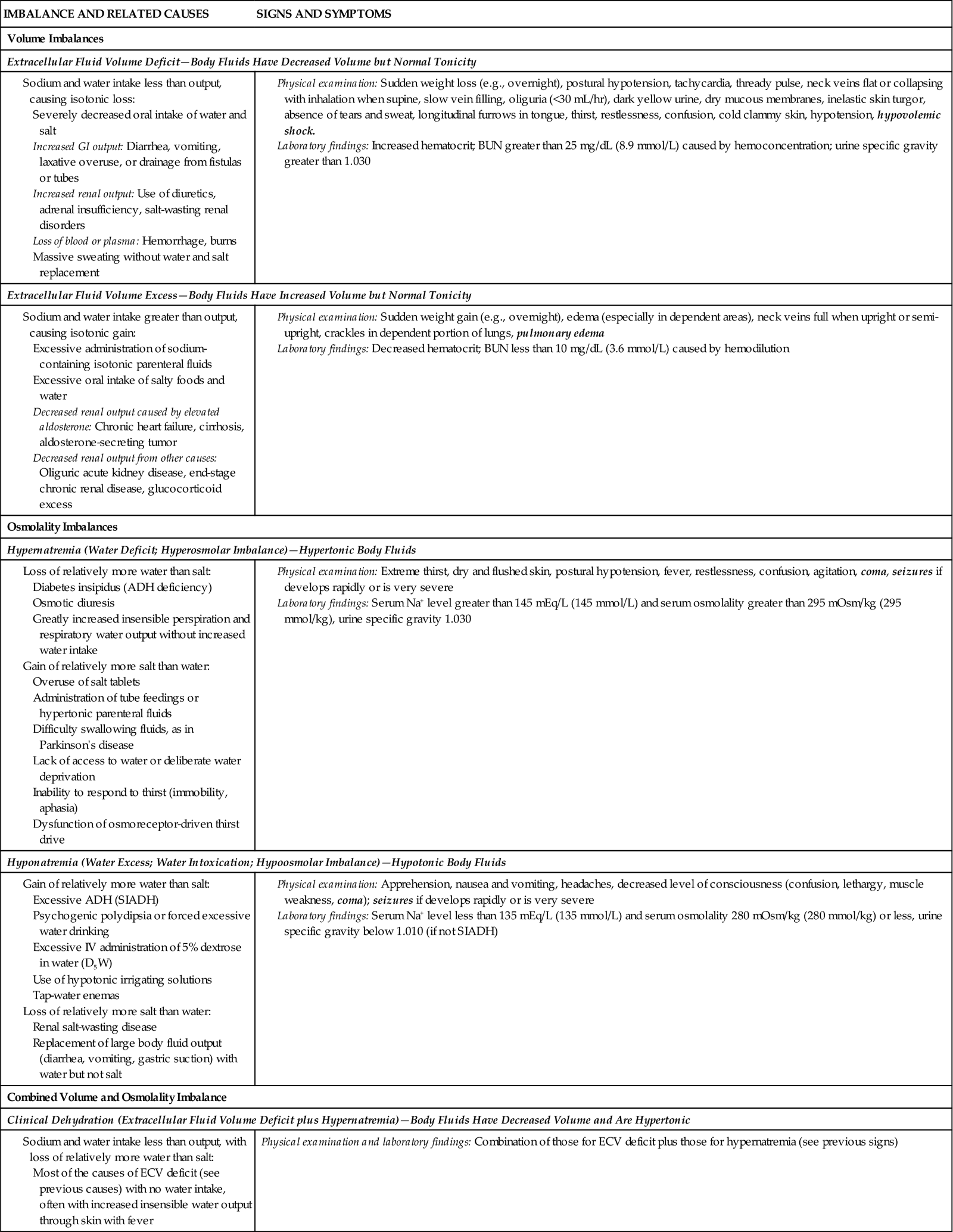
ADH, Antidiuretic hormone; BUN, blood urea nitrogen; ECV, extracellular fluid volume; GI, gastrointestinal; IV, intravenous; SIADH, syndrome of inappropriate secretion of antidiuretic hormone.
Text in bold italics denotes potentially life-threatening manifestations.
ECV excess occurs when there is too much isotonic fluid in the extracellular compartment. Intake of sodium-containing isotonic fluid has exceeded fluid output. For example, when you eat more salty foods than usual and drink water, you may notice that your ankles swell or rings on your fingers feel tight and you gain 2 lbs (1 kg) or more overnight. These are manifestations of mild ECV excess. See Table 41-3 for other specific causes and signs and symptoms.
Osmolality Imbalances.
In an osmolality imbalance body fluids become hypertonic or hypotonic, which causes osmotic shifts of water across cell membranes. The osmolality imbalances are called hypernatremia and hyponatremia.
Hypernatremia, also called water deficit, is a hypertonic condition. Two general causes make body fluids too concentrated: loss of relatively more water than salt or gain of relatively more salt than water (Felver, 2010b). Table 41-3 lists specific causes under these categories. When the interstitial fluid becomes hypertonic, water leaves cells by osmosis, and they shrivel. Signs and symptoms of hypernatremia are those of cerebral dysfunction, which arise when brain cells shrivel. Hypernatremia may occur in combination with ECV deficit; this combined disorder is called clinical dehydration.
Hyponatremia, also called water excess or water intoxication, is a hypotonic condition. It arises from gain of relatively more water than salt or loss of relatively more salt than water (Felver, 2010b) (see Table 41-3). The excessively dilute condition of interstitial fluid causes water to enter cells by osmosis, causing the cells to swell. Signs and symptoms of cerebral dysfunction occur when brain cells swell.
Clinical Dehydration.
ECV deficit and hypernatremia often occur at the same time; this combination is called clinical dehydration (Bryant, 2007). The ECV is too low, and the body fluids are too concentrated. Clinical dehydration is common with gastroenteritis or other causes of severe vomiting and diarrhea when people are not able to replace their fluid output with enough intake of dilute sodium-containing fluids. Signs and symptoms of clinical dehydration are those of both ECV deficit and hypernatremia (see Table 41-3).
Electrolyte Balance
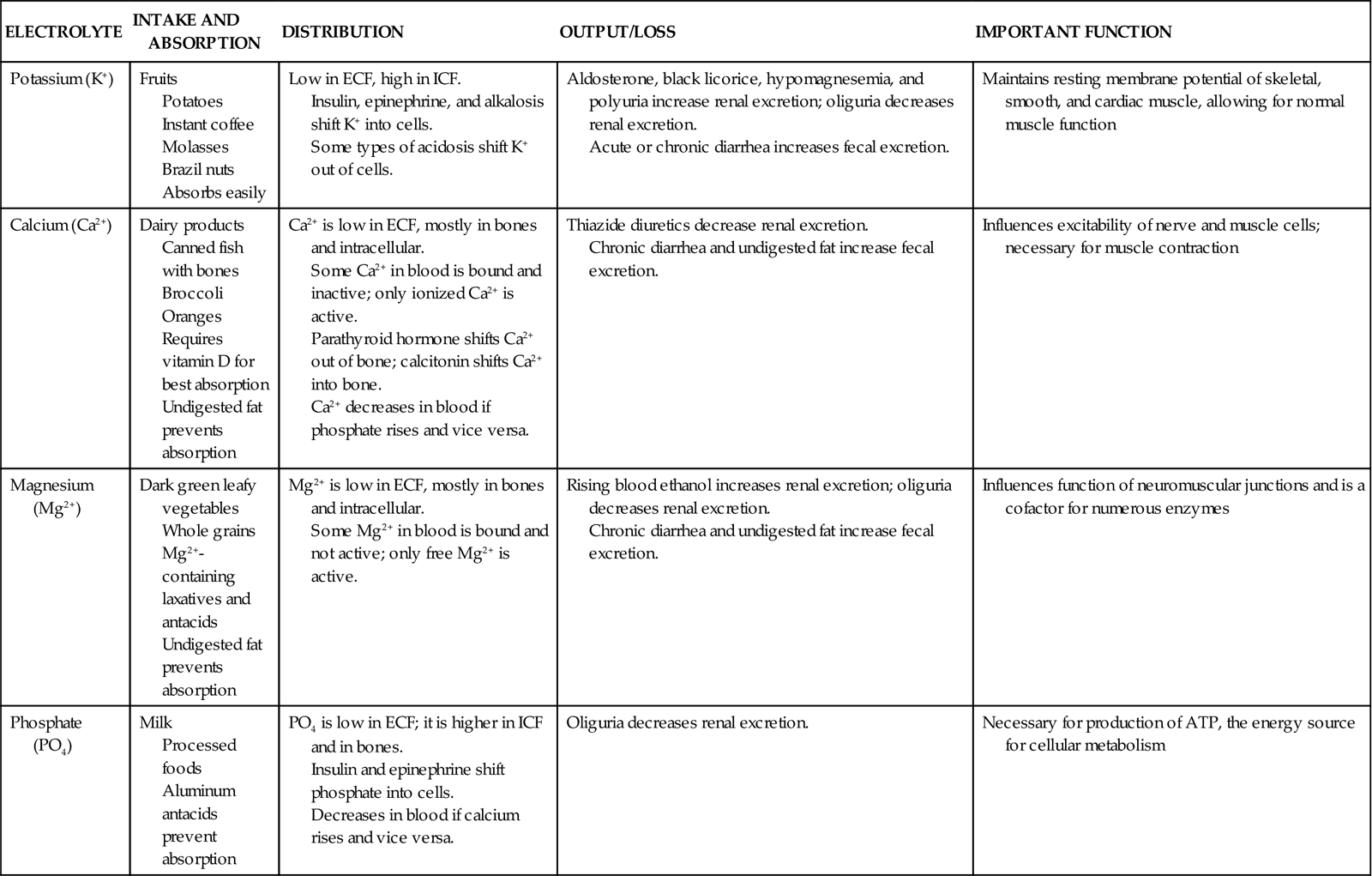
You can best understand electrolyte balance by considering the three processes involved in electrolyte homeostasis: electrolyte intake and absorption, electrolyte distribution, and electrolyte output (Table 41-4) (Felver, 2010b). Although sodium is an electrolyte, it is not included here because serum sodium imbalances are the osmolality imbalances discussed previously.
TABLE 41-4
Electrolyte Intake and Absorption, Distribution, and Output
| ELECTROLYTE | INTAKE AND ABSORPTION | DISTRIBUTION | OUTPUT/LOSS | IMPORTANT FUNCTION |
| Potassium (K+) | Fruits Potatoes Instant coffee Molasses Brazil nuts Absorbs easily | Low in ECF, high in ICF. Insulin, epinephrine, and alkalosis shift K+ into cells. Some types of acidosis shift K+ out of cells. | Aldosterone, black licorice, hypomagnesemia, and polyuria increase renal excretion; oliguria decreases renal excretion. Acute or chronic diarrhea increases fecal excretion. | Maintains resting membrane potential of skeletal, smooth, and cardiac muscle, allowing for normal muscle function |
| Calcium (Ca2+) | Dairy products Canned fish with bones Broccoli Oranges Requires vitamin D for best absorption Undigested fat prevents absorption | Ca2+ is low in ECF, mostly in bones and intracellular. Some Ca2+ in blood is bound and inactive; only ionized Ca2+ is active. Parathyroid hormone shifts Ca2+ out of bone; calcitonin shifts Ca2+ into bone. Ca2+ decreases in blood if phosphate rises and vice versa. | Thiazide diuretics decrease renal excretion. Chronic diarrhea and undigested fat increase fecal excretion. | Influences excitability of nerve and muscle cells; necessary for muscle contraction |
| Magnesium (Mg2+) | Dark green leafy vegetables Whole grains Mg2+-containing laxatives and antacids Undigested fat prevents absorption | Mg2+ is low in ECF, mostly in bones and intracellular. Some Mg2+ in blood is bound and not active; only free Mg2+ is active. | Rising blood ethanol increases renal excretion; oliguria decreases renal excretion. Chronic diarrhea and undigested fat increase fecal excretion. | Influences function of neuromuscular junctions and is a cofactor for numerous enzymes |
| Phosphate (PO4) | Milk Processed foods Aluminum antacids prevent absorption | PO4 is low in ECF; it is higher in ICF and in bones. Insulin and epinephrine shift phosphate into cells. Decreases in blood if calcium rises and vice versa. | Oliguria decreases renal excretion. | Necessary for production of ATP, the energy source for cellular metabolism |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree