Chapter 4 Fluid and Electrolytes
Insider’s Guide to Fluid and Electrolytes for The USMLE Step 1
Many of the important concepts that were touched upon in Chapter 3 are further explored in Chapters 4 and 5, on fluid and electrolytes and acid-base balance, respectively. Concepts relating to fluid and electrolytes make up a large portion of the renal physiology and pharmacology material tested on Step 1. You will soon see that this is not a section that requires memorizing lots of small details. Instead, it demands a thorough understanding of the mechanisms of action of various hormones and drugs involved in regulating fluid and electrolyte balance. Know which segments of the nephron are affected by these individual hormones and drugs. If applicable, it is a good idea to categorize how each hormone or drug affects glomerular filtration rate (GFR), renal plasma flow (RPF), filtration fraction, etc. For those of you who do not have a strong background in renal physiology, you may need to read through the discussion in this chapter a few times to fully grasp all of the material.
Basic concepts—renal filtration and transport processes
1 What forces govern the glomerular filtration rate at the level of the glomerulus?
These forces are the same forces that affect fluid movement in the systemic capillaries. Forces that drive fluid across the glomerular membrane include the hydrostatic pressure in the glomerular capillaries and the oncotic pressure in Bowman’s space (Fig. 4-1). Because there is usually very little protein in Bowman’s space, the contribution from the filtrate’s oncotic pressure is typically negligible. Forces that oppose fluid movement across the glomerular membrane are the hydrostatic pressure in Bowman’s space and the plasma oncotic pressure.
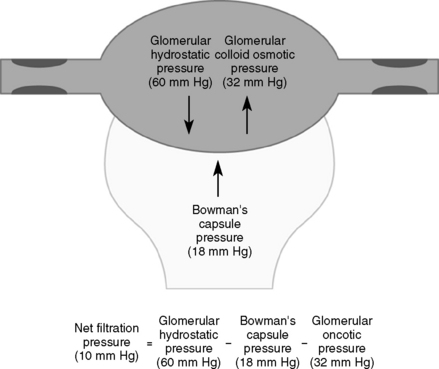
(From Guyton AC, Hall JE: Textbook of Medical Physiology, 11th ed. Philadelphia, WB Saunders, 2007.)
In addition to the oncotic and hydrostatic pressures, the surface area and integrity of the glomerular membranes are also important determinants of GFR. Mathematically, these factors are represented through a filtration constant. These factors are most relevant in disease states in which the glomeruli are damaged. The formula for calculating GFR is given in Chapter 3.
2 How do angiotensin-converting enzyme inhibitors and angiotensin receptor blockers affect glomerular filtration rate?
3 What is meant by the term filtration fraction and how will increasing the glomerular capillary oncotic pressure (without changing anything else) affect the filtration fraction?
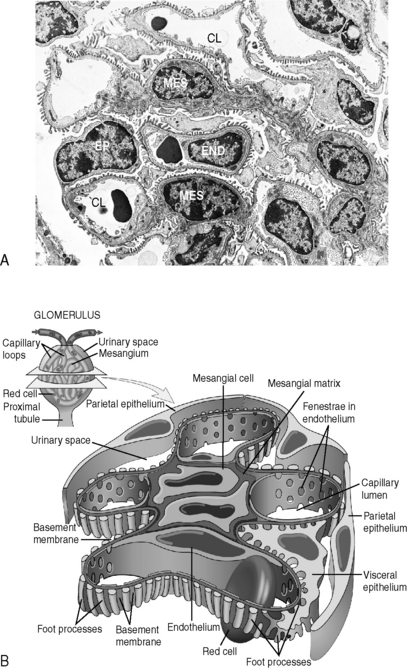
4 What are the three layers of the glomerular “filter” and how do they contribute to the process of renal filtration at the glomerulus?
Note: The glomerulus also contains macrophage-like mesangial cells and mesangial matrix interspersed between these layers (Fig. 4-2). The function of the mesangium is not very well understood (though it may serve both a structural role and a housekeeping role). Regardless, the mesangium can be an important site of glomerular disease.
5 What is the significance of the creatinine clearance and how is it measured?
Summary Box: General Concepts in Renal Filtration and Transport Processes
 The determinants of glomerular filtration rate (GFR) include the hydrostatic and oncotic pressures within the glomerular capillaries, the hydrostatic and oncotic pressures of Bowman’s space, and the collective glomerular surface area and integrity (i.e., filtration constant).
The determinants of glomerular filtration rate (GFR) include the hydrostatic and oncotic pressures within the glomerular capillaries, the hydrostatic and oncotic pressures of Bowman’s space, and the collective glomerular surface area and integrity (i.e., filtration constant).
 The hydrostatic pressure within the glomerular capillaries is regulated by vasoconstriction and vasodilation of the efferent and afferent arterioles.
The hydrostatic pressure within the glomerular capillaries is regulated by vasoconstriction and vasodilation of the efferent and afferent arterioles.
 Most circulating vasoactive substances act to constrict or dilate the afferent arteriole. Angiotensin II is unique in that it acts primarily to constrict the efferent arteriole.
Most circulating vasoactive substances act to constrict or dilate the afferent arteriole. Angiotensin II is unique in that it acts primarily to constrict the efferent arteriole.
 Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) may acutely decrease GFR, but their effect on lowering glomerular pressure helps preserve renal function over the long term in patients with chronic kidney disease.
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) may acutely decrease GFR, but their effect on lowering glomerular pressure helps preserve renal function over the long term in patients with chronic kidney disease.
 The three components of the glomerular filter are (1) the capillary endothelial cells, (2) the basement membrane, and (3) the epithelial cells, or podocytes, and the intervening mesangium.
The three components of the glomerular filter are (1) the capillary endothelial cells, (2) the basement membrane, and (3) the epithelial cells, or podocytes, and the intervening mesangium.
 Creatinine clearance is an important indicator of GFR. Clinically, it is usually estimated from a single measurement of serum creatinine concentration. However, recall that numerous variables (e.g. age, gender, muscle mass) can affect the creatinine concentration.
Creatinine clearance is an important indicator of GFR. Clinically, it is usually estimated from a single measurement of serum creatinine concentration. However, recall that numerous variables (e.g. age, gender, muscle mass) can affect the creatinine concentration.
 Estimates of creatinine clearance are inaccurate in the setting of a rapidly changing GFR (i.e., acute renal failure).
Estimates of creatinine clearance are inaccurate in the setting of a rapidly changing GFR (i.e., acute renal failure).
Basic concepts—renal control of acid-base balance
3 How do the kidneys reabsorb filtered bicarbonate?
Hydrogen ions that are secreted into the lumen of the proximal tubule (primarily via countertransport with Na+) react with bicarbonate in the filtrate to form carbonic acid, which rapidly dissociates into carbon dioxide and water, both of which can then diffuse back into the cell (Fig. 4-3). In the cell, the reverse reaction takes place. Thus, carbon dioxide reacts with water to generate bicarbonate and a proton (hydrogen ion). The bicarbonate is ultimately returned to the venous circulation, whereas the hydrogen ion is secreted back into the tubular lumen.
4 What effect does the diuretic acetazolamide have on the acid-base balance of the body? In what clinical conditions might it be used?
5 How are bicarbonate and ammonium generated de novo by the kidney?
The deamination of glutamine in the proximal tubule generates two ammonium (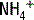 ) molecules and two bicarbonate (
) molecules and two bicarbonate (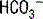 ) molecules. The ammonium molecules (which essentially consist of acidic protons being carried by ammonia) are secreted into the tubular lumen, whereas the basic bicarbonate molecules are reabsorbed into the systemic circulation (Fig. 4-4).
) molecules. The ammonium molecules (which essentially consist of acidic protons being carried by ammonia) are secreted into the tubular lumen, whereas the basic bicarbonate molecules are reabsorbed into the systemic circulation (Fig. 4-4).
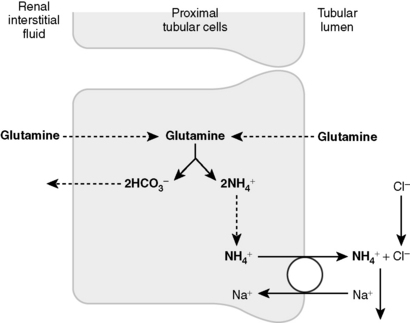
(From Guyton AC, Hall JE: Textbook of Medical Physiology, 11th ed. Philadelphia, WB Saunders, 2007.)
Summary Box: Renal Control of Acid-Base Balance
 The reabsorption of filtered bicarbonate in the proximal tubule via a mechanism requiring the activity of carbonic anhydrase
The reabsorption of filtered bicarbonate in the proximal tubule via a mechanism requiring the activity of carbonic anhydrase
 The de novo synthesis of bicarbonate (to be retained) and ammonium (to be secreted)
The de novo synthesis of bicarbonate (to be retained) and ammonium (to be secreted)
 The secretion of titratable buffers (e.g., ammonia, phosphate to increase the acid-carrying capacity of urine)
The secretion of titratable buffers (e.g., ammonia, phosphate to increase the acid-carrying capacity of urine)
 The secretion of acid via a proton pump in the distal tubule
The secretion of acid via a proton pump in the distal tubule
For more information on the regulation of acid-base balance, see Chapter 5.
Basic concepts—renal control of extracellular fluid balance
1 What are the extracellular fluid compartments of the body and how do their relative sizes compare to the intracellular fluid compartment?
The extracellular fluid (ECF) consists of the interstitial fluid and plasma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


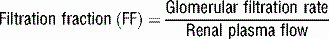


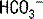 ) to be retained and the acid ammonium (
) to be retained and the acid ammonium (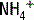 ) to be secreted, (3) secrete titratable buffers such as ammonia and phosphate (which bind hydrogen ions and increase the acid excretory capacity of the urine without causing a precipitous drop in urinary pH), and (4) actively pump acid (in the form of hydrogen ions) into the tubular fluid at the distal tubule.
) to be secreted, (3) secrete titratable buffers such as ammonia and phosphate (which bind hydrogen ions and increase the acid excretory capacity of the urine without causing a precipitous drop in urinary pH), and (4) actively pump acid (in the form of hydrogen ions) into the tubular fluid at the distal tubule.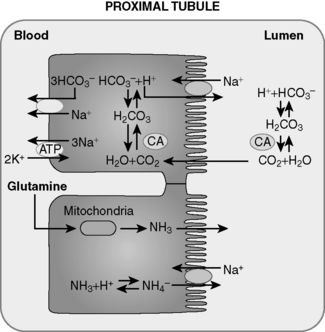
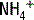 ) by proximal tubular cells. Glutamine is metabolized in the cell yielding
) by proximal tubular cells. Glutamine is metabolized in the cell yielding 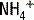 and bicarbonate. The
and bicarbonate. The 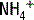 is actively secreted into the lumen by means of a sodium-
is actively secreted into the lumen by means of a sodium-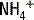 pump. For each glutamine molecule metabolized, two
pump. For each glutamine molecule metabolized, two 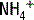 are produced and secreted and two
are produced and secreted and two 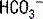 are returned to the blood.
are returned to the blood.