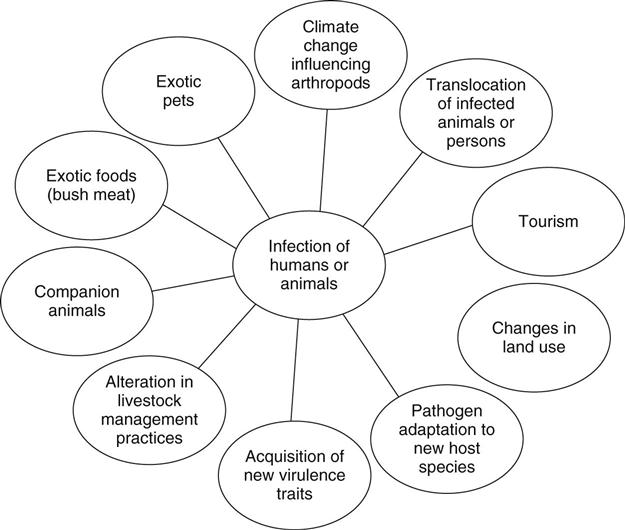
James Mark Simmerman “The deviation of man from the state in which he was originally placed by nature seems to have proven to him a prolific source of diseases.” —Edward Jenner The relationship between infectious disease and human population growth, poverty, urbanization, climate change, industrialized food production, and rapid travel is increasingly well documented. The continual threat to public health from emerging and reemerging infectious diseases (EID) demands sustained attention, international cooperation, and resources. Innovative approaches are needed to improve the laboratory detection, surveillance systems, and control of EIDs in the context of rapidly changing human and pathogen ecology. In 1962, immunologist and Nobel laureate Sir MacFarlane Burnet wrote that the middle of the twentieth century could be regarded as the end of one of the most important social revolutions in history, reflecting the virtual elimination of infectious disease as a significant factor in social life (Burnet, 1962). Indeed, the use of antibiotics, immunizations, and improved public health systems had significantly reduced deaths from infectious diseases in the United States. However, beginning in the early 1980s, human immunodeficiency virus infection and acquired immunodeficiency syndrome (HIV/AIDS) together with opportunistic infections such as multidrug-resistant tuberculosis, refocused global attention on the threat from emerging infectious diseases (Patton et al., 2009). Despite progress toward prevention and treatment, HIV/AIDS is still estimated to become the leading cause of disease burden in middle- and low-income countries by 2015 with 6.5 million deaths projected in 2030, even under the assumption that coverage with antiretroviral drugs reaches 80% (Mathers & Loncar, 2006). Outbreaks of other emerging pathogens have also occurred with increasing frequency. The majority (60%) of EID events are caused by zoonotic pathogens (a non-human animal source), and 72% of these events are caused by pathogens originating from wildlife (Jones et al., 2008). These pathogens can change hosts as a result of multiple complex factors that often work in combination (Cutler, Fooks, & Van Der Poel, 2010) (Figure 103-1). In 1992, a report by the Institute of Medicine (IOM) called attention to the global problem of emerging infectious diseases (Lederberg, Shope, & Oaks, 1992). This was followed by two reports from the Centers for Disease Control and Prevention (CDC) that further characterized the issues (CDC, 1994, 1998). Emerging infectious diseases were defined as new, reemerging, or drug-resistant infections whose incidence in humans have increased within the past two decades or threatened to increase in the near future. Reemerging diseases refers to the reappearance of a known disease following a decline in incidence and (Lederberg et al., 1992) include newly recognized pathogens, new diseases caused by known organisms, and the extension of the geographic or host range of a pathogen (Lashley, 2003, 2004). The factors listed in Box 103-1 often operate in concert and have been associated with the emergence or reemergence of infectious diseases. Since 1980, the emergence or reemergence of infectious diseases has had a significant impact on global health and economies (Binder, Levitt, Sacks, & Hughes, 1999; Morens et al., 2004). The speed and expansion of modern international air travel and global trade place every country at risk and require all to contribute to surveillance and control efforts.Recent examples of EID outbreaks include Avian Influenza A (H5N1), West Nile virus, SARS coronavirus, and Monkey pox. The first confirmed case of avian influenza A (H5N1) infection identified in a human occurred in Hong Kong in May 1997. Six months later in November and December 1997 an additional 17 infections were detected (Chan 2002; Bridges et al., 2002). In 2001, the virus reappeared in humans in Hong Kong, and in late 2003 large-scale poultry die-offs and new human infections occurred in several countries in East and Southeast Asia (Uyeki, 2008). By 2006, the virus had become endemic in most of East and Southeast Asian poultry, and human avian influenza infections had been documented in Eastern Europe and several African countries. While infections are often fatal, transmission from infected poultry to humans remains inefficient, and only limited human-to-human transmission has been observed (Ungchusak et al., 2005; Kandun et al., 2006). By October 2010, the World Health Organization (WHO) reported 507 confirmed cases with 302 deaths in 15 countries worldwide (WHO, 2010). Concern that the H5N1 virus could develop the ability to efficiently pass from person to person has led to the establishment of an aggressive global research and training agenda addressing surveillance, prevention, pandemic preparedness, epidemiological investigation, laboratory diagnostics, molecular virology, pathogenesis, and vaccine development (Oshitani, Kamigaki, & Suzuki, 2008; WHO, 2005a; Knobler, Mack, Mahmoud, & Lemon, 2005). Emerging infectious diseases are increasingly due to the expanded geographic range of insect vectors. While vector-borne disease has long been a major cause of disability in poor, tropical countries, with the recent arrival of the West Nile virus it has become an important public health issue in temperate North America (Griffin, 2009). West Nile virus, a long-recognized cause of encephalitis in Africa and the Middle East, was first recognized in North America in 1999 (Hughes, 2001). Since that time, West Nile virus spread across the Western hemisphere and has become (Granwehr et al., 2004) the leading cause of arboviral disease in the U.S. While an estimated 80% of infections are asymptomatic, symptomatic persons develop an acute febrile illness that includes headache, myalgia, arthralgia, rash, or gastrointestinal symptoms. Neuroinvasive disease typically presents as encephalitis, meningitis, or acute flaccid paralysis. Between 1999 and 2008, the U.S. CDC National Electronic Passive Surveillance System recorded a total of 28,961 confirmed and probable cases of West Nile virus disease, including 11,822 (41%) neuroinvasive cases from 47 states (Lindsey et al., 2009). In the absence of an effective human vaccine, West Nile virus prevention depends on community-level mosquito control and household and personal protection measures. The coronavirus associated with severe acute respiratory syndrome (SARS) first emerged in southern China in late 2002. A traveler who was incubating SARS traveled from Guangdong province to Hong Kong and subsequently infected other hotel guests. These individuals then traveled to other countries, seeding outbreaks in at least 26 countries (Christian, Poutanen, Loutfy, Muller, & Low, 2004; Skowronski et al., 2005). As the number of SARS cases increased in early 2003, the WHO and the CDC issued travel advisories (WHO, 2003). In just a few months, major economic losses resulted from curtailed business and tourist travel to various cities in East Asia as well as in Toronto, Canada. In all, 8096 cases were recorded with 774 deaths in 29 countries. For more information, visit www.who.int/csr/sars/country/table2004_04_21/en/index.html. The SARS outbreak was characterized by severe nosocomial outbreaks, often involving nurses, and the unprecedented and rapid international cooperation among the world’s most sophisticated laboratories in order to identify the new viral pathogen (Simmerman 2003; Cheung et al. 2005). The SARS outbreak was contained in July 2003, but additional cases of infection have been reported in laboratory scientists studying the virus (Christian et al., 2004; Poon, Guan, Nicholls, Yuen, & Peiris, 2004). Trade in domestic and exotic animals for food or pets has become an increasingly common source of infectious disease outbreaks. Monkey pox is an orthopox virus closely related to smallpox viruses. The virus was recognized in 1970 as a human pathogen in tropical Africa responsible for sporadic infections in rainforest hunters and rare outbreaks involving human-to-human transmission. Monkey pox was not considered to be a threat outside Africauntil 2003, when 82 children and adults in 11 Midwestern U.S. states contracted the infection. The outbreak resulted from contact with prairie dogs imported as exotic pets that had beeninfected during transport in cages with with Gambian giant rats (Reed et al., 2004; CDC, 2003b). An embargo on the import, sale, and transport of rodents from Africa and restrictions on the sale or movement of prairie dogs was announced in June 2003, and no cases have since been detected in North America (CDC, 2003a). Novel influenza A virus strains appear at unpredictable intervals and can cause outbreaks on a global scale (pandemics) with sharply increased morbidity and mortality, especially in children and young adults (Simonsen et al., 1998; Monto, 2009). Pigs are susceptible to infection from influenza viruses of both avian and human origins, efficiently transmit infection, and play an important role in the generation of novel influenza viruses (Ma et al., 2009). In early 2009, a multiple reassortant (with genes from swine, human, and avian viruses) influenza A/H1N1 virus emerged in southern Mexico (Brownstein, Freifeld, & Madoff, 2009). Beginning in late February 2009, national surveillance systems in Mexico detected an unusual increase in influenza-like illness (ILI) cases, and in March and April there were increasing reports of previously healthy young adults hospitalized with severe pneumonia. This led to active surveillance in 23 hospitals in Mexico City and the identification of non-subtypable influenza A viruses (Perez-Padilla et al., 2009). In March 2009, two epidemiologically unrelated children in southern California were detected that had been infected with the new reassortant virus, but neither had had contact with pigs. This set off national alerts and the establishment of syndromic surveillance systems in schools, cities, and states across the country. In New York, the first indication of an outbreak came from a telephone report from a school nurse reporting an increase in febrile illness after students returned from spring break vacation to Mexico. This novel virus rapidly spread around the world causing the WHO to declare a pandemic on June 11, 2009 (Novel Swine-Origin Influenza A [H1N1] Virus Investigation Team et al., 2009; WHO, 2009). Fortunately, while the 2009 pandemic influenza A (H1N1) virus resulted in millions of illnesses, caused significant social disruption, and increased burden on health care systems, mortality rates from this virus were lower than that observed during previous pandemics. Among the major policy domains associated with emerging and reemerging infectious disease outbreaks are surveillance and reporting, immunization, quarantine, travel and immigration restrictions, and restrictions related to the importation of food and animals. The International Health Regulations (IHRs) were adopted in 1969, amended in 1973 and 1981, and completely revised in 2005 at the 59th World Health Assembly to provide a legal framework for international cooperation. The main purpose of the IHRs is to prevent, control, and provide a public health response to the international spread of disease in ways that avoid unnecessary interference with international traffic and trade. The 2005 revision is legally binding on WHO member states and came into effect in 2007. This revision broadened the scope of the IHR from focusing on cholera, plague, and yellow fever to include any public health event of international concern (PHEIC). Under the IHRs, all WHO member states are required to develop, strengthen, and maintain core surveillance and response capacities, facilitate cross-border cooperation, and provide logistical and financial support to improve capacity to conduct surveillance and response activities (WHO, 2005b). The IHRs promote improved coordination with agricultural authorities such as the Food and Agriculture Organization (FAO) and the World Organization for Animal Health (OIE), to reduce the potential for EID outbreaks from food, livestock, and wild animal sources (Pavlin, Schloegel, & Daszak, 2009; Newell et al., 2010). The WHO also conducts a variety of global and regional surveillance activities under the umbrella of Communicable Disease Surveillance and Response (CSR), including the Global Outbreak Alert and Response Network (GOARN), which monitors communicable diseases as well as food and water safety. Surveillance for infectious disease relies on formal and informal systems and may be syndromic or pathogen specific, depending on the objectives and available resources. Examples of these systems include the following: • The U.S. CDC mandates reporting from the states for nationally notifiable diseases (http://cdc.gov/mmwr/PDF/wk/mm5653.pdf) through the National Notifiable Disease Surveillance System (NNDSS); reports are transmitted through the National Electronic Telecommunications System for Surveillance (NETSS). • The National Healthcare Safety Network (NHSN) (www.cdc.gov/nhsn) is a voluntary, secure, Internet-based surveillance system that integrates health care personnel safety surveillance systems managed by the Division of Healthcare Quality Promotion (DHQP) at the CDC to provide data on emerging healthcare-associated infection and pathogens, assess the importance of potential risk factors, characterize their mechanisms of resistance, and evaluate alternative surveillance and prevention strategies in U.S. health care facilities. The Surveillance of Emerging Antimicrobial Resistance Connected to Healthcare (SEARCH) system is a network of hospitals and health departments that conducts surveillance on antibiotic-resistant Staphylococcus aureus. • The Global Public Health Intelligence Network (GHPIN) (www.phac-aspc.gc.ca/media/nr-rp/2004/2004_gphin-rmispbk-eng.php) is a secure, Internet-based “early warning” system that gathers preliminary reports of public health significance in seven languages on a real-time, 24/7 basis. GHPIN is conducted by the Public Health Agency of Canada and focuses on chemical, biological, radiological, and nuclear public health threats throughout the world.
Emerging and Reemerging Infectious Disease
A Global Challenge
Background
Defining Emerging and Reemerging Infections
A Global Concern
Avian Influenza A (H5n1)
West Nile Virus
Sars Coronavirus
Monkey Pox
2009 Pandemic H1N1 Influenza
Global Surveillance and Reporting
Surveillance and Reporting Systems
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Nurse Key
Fastest Nurse Insight Engine
Get Clinical Tree app for offline access
