Chapter 29 Fertilization, embryonic, fetal and placental development
After reading this chapter, you will be able to:
Introduction
This chapter will build on Chapters 24, 25 and 27. The luteal phase of the fertile cycle initiates very distinct trajectories for mother and conceptus (see website). Following the luteinizing hormone/follicle-stimulating hormone (LH/FSH) surge, hormonal signals from the corpus luteum induce extensive adaptations in maternal renal, cardiovascular, respiratory, immunological, metabolic, endometrial and mammary systems. When fertilization occurs, these accelerate in response to signals from the conceptus and the endometrium. Before and after implantation, paracrine ‘cross-talk’ between conceptus and the endometrium prepares for attachment, implantation, differentiation of the trophoblast and development of the conceptus. At the same time, neuroendocrine signals are relayed from conceptus to the pituitary–ovarian and pituitary–thyroid regulatory axes; the peripheral immune system and maternal brain. These extend the functional lifespan of the corpus luteum; induce adaptations in maternal thyroid, stress and immune systems; and alter maternal sleep–wake cycles and food preferences, to accommodate the fertile cycle.
Fertilization
Spontaneous fertilization requires synchronized maturation and transport of the cumulus–oocyte complex (COC) and thousands of spermatozoa, within a tight timeframe. Once the COC is picked up by fimbriae of the fallopian tube, the oocyte has an estimated lifespan of 6–24 hours and spermatozoa 24–48 hours following arrival in the vaginal cavity (Johnson 2007:176). Both cells rapidly undergo a series of developmental and maturational processes, ensuring that only one of the 2–4 million spermatozoa that arrive in the vagina during sexual intercourse acquires the capacity to fuse with the oocyte membrane and release the sperm nucleus into the oocyte cytoplasm (Evans 2002, Kaji & Kudo 2004, Talbot et al 2003).
The fallopian tubes
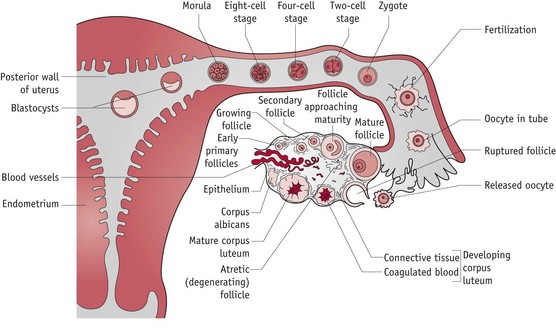
The fallopian tubes have critical functions following ovulation. The inner linings and secretions facilitate bi-directional transport of oocyte and spermatozoa; create favourable conditions for oocyte maturation, sperm storage, capacitation, fertilization and successive cleavage of the fertilized egg; and aid its transport towards the designated implantation site in the uterus (Leese et al 2001, Shafik et al 2005) (Fig. 29.1).
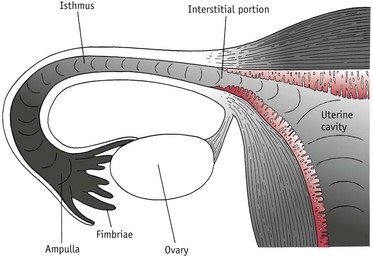
Figure 29.1 Female reproductive tract.
(Reproduced with permission from Carlson 1994:15; Fig. 1-13.)
Anatomically part of the uterus, inner layers of the fallopian tubes are continuous with those in the cavity. They are composed of an internal mucosa of ciliated and secretory epithelium sensitive to changes in pituitary gonadotrophins and ovarian steroids, across the cycle (Casan et al 2000, Lei et al 1993). Intermediate layers of smooth muscle contain blood, lymph vessels, and steroid-sensitive adrenergic neurons that enable coordinated tubular motility, for the journey of the blastocyst to the uterus (Habayeb et al 2008; Wang et al 2004, 2006).
Around ovulation, smooth muscles display characteristic movements that bring the infundibulum, or distal portion of the tube, into apposition with the ovary containing the dominant follicle, by a change in orientation of muscles surrounding the ovarian fimbriae (Hunter 1988, Zervomanolakis et al 2009). One fimbria – slightly longer than the rest – reaches out to the tubal pole of the ovary and involutes, in synchrony with ovulation, to pick up the COC from the peritoneal cavity, into the enlarged trumpet-shaped infundibulum, lined with a very dense layer of ciliated secretory epithelium.
Oocyte and blastocyst transport within the tube is facilitated by:
The interstitial portion is continuous with the uterine cavity and characterized by a marked increase in ciliated cells, and alterations in the shape of secretory cells. Muscles at the uterotubal junction are formed from four bundles. These hormonally sensitive interlacing spiral fibres allow strong constriction and relaxation of the interstitial portion of the tube. This regulates sperm transport and storage, and movement of the blastocyst towards the uterine cavity (Hunter 1988, Wildt et al 1998).
Cyclical changes
Across the ovarian cycle, the tubular mucosa undergoes cyclical alterations similar to the endometrial lining of the uterus. In the first half of the cycle, secretory and ciliated cells become larger under the influence of oestrogens. Around ovulation, ciliated cells become broader and lower while secretory cells become more distended with fluid. Following ovulation, microscopic holes appear in secretory cell membranes, which coalesce to release secretions accumulated during the first half of the cycle (Hunter 1988).
Higher rates of ciliary movements within the tube ipsilateral to the dominant follicle are regulated by increased blood flow, higher temperature and higher concentrations of ovarian steroids, compared to the contralateral tube (Zervomanola et al 2009). Around ovulation, beating of the dense concentration of cilia in the fimbriated portion is closely synchronized, propelling the COC into the ampulla. During this period, cilia in the ampulla also beat in the direction of the isthmus, suggesting that they further propel the COC towards the site of fertilization, taking the newly fertilized egg and surrounding cells from the ampulla and aiding subsequent movements of the zygote towards endometrial implantation (Hunter 1988).
The tubular environment is highly responsive to changing metabolic needs of the oocyte and blastocyst, through rhythms in secretion of growth factors, antioxidant enzymes and other regulatory molecules, and the supply of chemical and nutritive factors within the fallopian tubes following ovulation (Lapointe et al 2005). The oocyte and blastocyst are exposed to rhythmic secretion of growth regulatory molecules, and precisely timed modulations occur in oxygen tension, glucose concentrations, electrolytes and macromolecules, in different parts of the tube. The composition of tubular fluid complements the low rate of metabolite consumption in the oocyte and newly fertilized egg (Fleming et al 2004, Leese 1995, 2002, Leese et al 2001).
Both demonstrate optimal functioning in an alkaline environment, with low concentrations of glucose and oxygen and plentiful supplies of albumin and growth factors (Leese 1995). The nutritive environment of the tubular lumen is regulated by hormonally induced secretions of the tubular mucosa and metabolic activities of the cumulus cell mass which surrounds the oocyte and blastocyst until implantation (Leese et al 2001) (Fig. 29.2).
Sperm transport
Spermatozoa enter the genital tract in approximately 3–4 mL of seminal fluid which buffers the acidity of vaginal secretions. Over 99% are immediately lost by leakage from the vagina. Those remaining spend variable times in the cervix, showing differential states of motility and rates of transport through the uterus which may increase the chances of fertilization. This reservoir of spermatozoa in the cervix are actively transported in successive waves of peristalsis to a second sperm reservoir site in the isthmus, and finally to the fertilization site, at the isthmic–ampullary junction (Bahat et al 2003, Kunz et al 1998, Wildt et al 1998, Zervomanolakis et al 2009).
Cyclical changes in the vaginal canal around ovulation provide a protected environment for ejaculated sperm. This is mirrored by cyclical changes in cervical and vaginal tissues, including relaxation and widening of the cervix, and an increase in the quantity of cervical mucus, which demonstrates a characteristic ‘stretchiness’ that facilitates sperm transport (Drobnis & Overstreet 1992).
Sperm capacitation
During their active transport through the genital tract, spermatozoa undergo a final series of maturational changes before a small number are ready for fertilization. The first of these is capacitation, caused by interactions between spermatozoa and secretions of the cervix, uterus and fallopian tube during their journey to the site of fertilization. Capacitation is when the composition of the cell membrane undergoes removal of most glycoprotein molecules added during ejaculation. Capitation seems to induce hyperactive motility, providing increased thrusting power and alterations in cell surface properties enabling entry to the zona pellucida surrounding the oocyte cell membrane (Alberts et al 2002, Drobnis & Overstreet 1992). Sperm also acquire:
Fusion of oocyte and spermatozoon
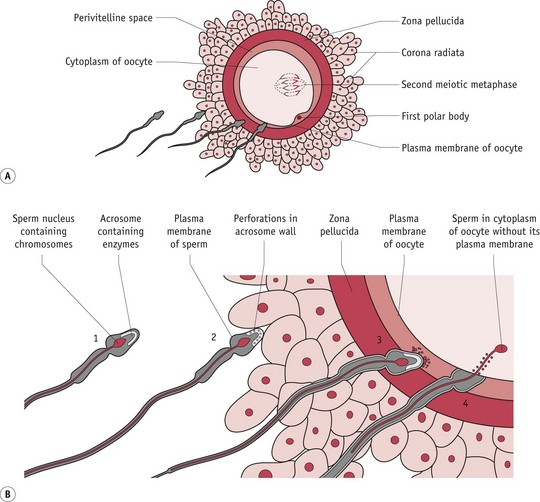
The final set of morphological transformations in spermatozoa are stimulated by binding to the zona pellucida. During this critical process, the acrosome swells and its membranes fuse with the overlying plasma membrane (see Fig. 29.3). Initially, attachment is very loose, involving a number of spermatozoa. Firmer binding follows as an oocyte-binding protein on the sperm head region is recognized by sperm receptors on the zona pellucida. The inner plasma membrane at the apical end of the spermatozoa fuses with the outer membrane of the acrosome and forms a series of membrane-bound vesicles. Proteolytic enzymes released by the acrosome reaction digest sections of the zona pellucida surrounding the sperm head. Subsequent movement through the zona occurs very rapidly, creating immediate access to the oocyte membrane. Only spermatozoa that have undergone this acrosomal exocytosis can fuse with the oocyte. Usually, the spermatozoon that makes first contact with the oocyte proceeds to fertilization. In the process of fusion, the plasma membrane of the sperm head is enveloped by microvilli on the oocyte surface (Johnson 2007, Tosti & Boni 2004).
From zygote to …
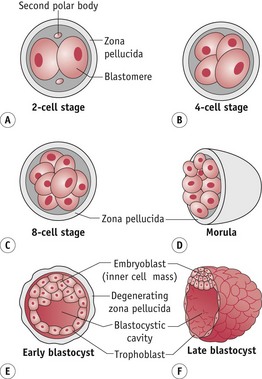
The zygote is the newly formed cell containing maternal and paternal chromosomes and measures about 0.15 mm in diameter (FitzGerald & FitzGerald 1994:12). Within 2–3 hours of fertilization, the zygote proceeds with the final phase of meiosis that was halted immediately following fertilization, transmitting one set of chromosomes to the next generation. The remaining set is discarded to a second polar body, on the periphery, which later undergoes apoptosis, along with the first one formed at ovulation (Fig. 29.3). Female chromosomes then divide mitotically, yielding one haploid set within the main body of the cytoplasm.
The cytoplasmic content of the sperm cell membrane combines with that of the oocyte and over the next 2–3 hours the sperm nuclear membrane gradually breaks down. Between 4 and 7 hours after cell fusion, two sets of haploid chromosomes are formed into male and female pronuclei, as each becomes surrounded by distinct membranes, in opposite poles of the cell. During this period, chromosomes synthesize DNA in preparation for the first mitotic division. As chromosomal content increases, the pronuclear membranes break down, bringing together two sets of male and female chromosomes. These events form the diploid complement of a new individual and the cell immediately proceeds with a first mitotic division, passing its genetic material to two daughter cells and forming the two-cell conceptus (Downs 2008) (Fig. 29.4).
… morula
Successive rounds of cleavages occur at approximately 12-hour intervals until 8–16 increasingly smaller cells are formed within the zona pellucida and remaining fragments of the cumulus cell mass. When cell cleavage has produced 8–16 cells, the conceptus changes its morphology and undergoes compaction to become a morula (resembling a mulberry) (Fig. 29.4). This process maximizes contiguity between adjacent cell membranes (see website).
Glucose is consumed in increasing quantities and the morula utilizes a large number of growth factors for replication (Leppens-Luisier et al 2001), including epidermal growth factor (EGF), insulin-like growth factors (IGFs), and hypoxia-inducible factors (HIFs) (Leese 1995). All blastomeres show intense staining for gonadotrophin-releasing hormone (GnRH), in possible readiness to stimulate human chorionic gonadotrophin (hCG) release just before implantation (Casan et al 1999, Kikkawa et al 2002). During this phase of formation, the morula remains within the zona pellucida. This smooth outer covering provides an overall structure preventing premature adhesion of the blastomeres to the wall of the fallopian tube and providing an immunological barrier between maternal tissue and the genetically distinct cells of the morula (Johnson 2007).
… to blastocyst
Over 24 hours, the morula continues cell cleavage to 16–32 cells. Between these two points, outer cells commit to trophoblast lineage, while the inner cell mass (ICM) retains the capacity for pluripotency. Expression of critical genes for blastocyst formation commences in the 16-cell morula (Armant 2005). Outer cells begin to pump fluid internally to form a fluid-filled cavity called the blastocoele. Nudged to one side by this fluid, the ICM expresses gap junctions, allowing transfer of ions and small molecules from one cell to the next (Downs 2008). Meanwhile, the outer layer underlying the zona pellucida differentiates into flattened trophectoderm cells that combine with the zona to protect the ICM from destruction by maternal immune cells and signals the endometrium to initiate adhesion (Schultz 1998).
The fertilized egg has now become a blastocyst (Fig. 29.4). The trophoectoderm expresses mRNA for leptin and both outer and inner cells express mRNA, protein and receptors for GnRH, and the cannabinoid receptor (CB1) (Battista et al 2008, Casan et al 1999).
Composed of 34–64 cells, the blastocyst is programmed to prepare for the embryonic phase of formation, beginning as a free-living organism immersed in uterine secretions actively accumulated by the trophoblasts and utilized as metabolic substrates, while exchange of oxygen and carbon dioxide occurs by diffusion (Burton et al 2002, Leese 1995). Over the next 3 days, paracrine cross-talk between the blastocyst and endometrium coordinates blastocyst activation with differentiation of the endometrium to a receptive state, from 7–9 days following ovulation (Fitzgerald et al 2008, Simon et al 1997).
The corpus luteum
During the fertile cycle, the functional lifespan of the corpus luteum extends from 14 to around 280 days, but the endocrine–paracrine regulation is not well understood, particularly after the first 6 weeks of pregnancy (Handschuh et al 2007, Muyan & Boime 1997, Oon & Johnson 2000).
Relaxin
Following the LH/FSH surges, large luteal cells (LLCs) and small luteal cells (SLCs) develop the capacity to secrete peptide and steroid hormones, in equal amounts. Relaxin, a peptide hormone of the insulin-like growth factor family, is a major hormone of the corpus luteum of pregnancy. Beginning during the luteal phase, under the stimulatory influence of LH/hCG, relaxin secretion peaks at 10 weeks’ gestation, decreases by around 20% and is present in maternal plasma, at stable concentrations, for the remainder of pregnancy (Bell et al 1987). Ovarian relaxin:
Hormonal signals
Within a week of fertilization, intact hCG (see website) appears in the maternal circulation and rapidly increases in the first 4 weeks after implantation, reaching peak levels of more than 100,000 mIU/mL around 9–10 weeks’ gestation, before falling rapidly to levels under 50,000 mIU/mL from around 18–20 weeks, until the end of pregnancy (Chard et al 1995, Kosaka et al 2002). In contrast, α-hCG levels increase progressively until term (Nagy et al 1994). At present, the exact mechanisms regulating intact hCG secretion are not well understood. Placental GnRH and leptin regulate secretion for the first 8 weeks of pregnancy (Islami et al 2003). Thereafter, hCG secretion as well as uterine and placental expression of LH/hCG receptors seem to be maintained by an autoregulatory mechanism (Cameo et al 2004, Kikkawa et al 2002). As pregnancy progresses, rising levels of fetal adrenal steroids seem to inhibit placental hCG (Tsakiri et al 2002).
hCG appears to have a fundamental role in regulating the establishment, development and maintenance of human pregnancy and the onset of labour (Ambrus & Rao 1994, Handschuh et al 2007, Ticconi et al 2007). Before attachment, trophectoderm cells of the blastocyst secrete hCG (Lopata et al 1997). Following attachment, hCG is released from mitotically active mononuclear cytotrophoblast cells, multinuclear syncytiotrophoblasts and extravillous cytotrophoblasts that differentiate from the trophoblast following implantation (Handschuh et al 2007, Muyan & Boime 1997). This early pregnancy rise in hCG secretion by the syncytiotrophoblast, extraplacental chorion and endometrial lining of the uterus and fallopian tubes constitutes the first neuroendocrine signal that induces maternal recognition of the fertile cycle (Handschuh et al 2007).
Preparation for implantation
In readiness for implantation, endometrial glands accumulate glycogen, proteins, a variety of growth factors, sugars and lipid droplets, and secretory activity peaks at around 6 days after ovulation (Burton et al 2002, 2007). Some secretions supply the conceptus with essential nutritional and regulatory molecules until around 9 weeks’ gestation, while growth factors stimulate proliferation of cytotrophoblast cells following implantation and secretion of hCG and human placental lactogen (hPL) by the syncytiotrophoblast from 6 to 12 weeks (Burton et al 2007, Hustin & Franchimont 1992, Jauniaux et al 2003).
Ovarian progesterone stimulates increased expression of two potent angiogenic factors: angiogenin in stromal cells and vascular endothelial growth factor (VEGF) in stromal cells and neutrophils associated with microvessel walls (Gargett et al 2001, Ma et al 2001). By the mid-secretory phase, a subepithelial capillary plexus has formed into a complex network of vessels (see Web Fig. 29.1) and newly regrown arterioles become increasingly spiral as they lengthen more rapidly than the endometrium thickens (Gargett et al 2001, Starkey 1993, Strauss & Coutifaris 1999).
Blastocyst–endometrial communication
When fertilization occurs, the designated attachment site undergoes extensive synchronized changes transforming it from a usual state of active rejection of blastocyst attachment, to a brief state of receptivity, forming a ‘window of implantation’ commencing 7 days after ovulation (Fitzgerald et al 2008, Hustin 1992, Hustin & Franchimont 1992, Lessey 2000). Some changes are regulated by paracrine cross-talk with the free floating blastocyst, while others are activated by attachment.
As the blastocyst hatches from the zona pellucida, around 6 days after fertilization, trophoblast cells surrounding the ICM make initial contact with the endometrium, by close apposition of trophoblast plasma membranes with the apical membranes of surface epithelial cells, followed by rapid proliferation and formation of junctional complexes with the surface epithelium. Apical membranes of epithelial cells display a variety of progesterone- and oestrogen-induced changes that facilitate cell recognition and interaction with the trophectoderm. These include a progressive shortening of the microvilli, creating a flatter surface, reduced thickness of the normally dense coating of glycoproteins, and inhibition of gap junctions, facilitating adhesion (Fride 2008, Hustin & Franchimont 1992, Johnson 2007, Lessey 2000).
Adhesion and attachment
Around the designated site of implantation, the luminal epithelium expresses calcitonin and the blastocyst-dependent heparin-binding epidermal growth factor (HB-EGF). As the zona pellucida is shed, direct communication between cell adhesion molecules and their receptors on trophoblast and surface epithelium begins the dynamically balanced processes of attachment, implantation and trophoblast replication (Fitzgerald et al 2008) (see website).
Implantation – endometrial response
The surface epithelium, uterine glands and decidua show distinct responses to implantation. As the tiny blastocyst lodges in the crypt of the endometrial folds and localized oedema moulds a chamber around the trophoblast surface, epithelial cells above the newly created site multiply rapidly to form a complete cover for the embedded blastocyst enveloped in a growing mantle of differentiating trophoblast cells, by approximately 9 days following fertilization (Armant 2005). More extensive hormone-induced changes occur within the underlying decidua, producing a range of matrix proteins to form a loose lattice-type network allowing free passage of water, ions and large molecules to the trophoblast cells.
Like epithelial glands, decidual cells synthesize and release specific glycoproteins. One of these has been identified as a growth factor-binding protein that may participate in regulating the pace and extent of trophoblast implantation (Hustin & Franchimont 1992). Decidual cells that release relaxin have also been found to contain prolactin, which is regulated by a number of factors from adjoining cells in the decidua, placenta and membranes. Activities of decidual prolactin include regulation of glandular secretions, expression of adhesion and proteolytic molecules, and modulation of specific aspects of the immune response (Gubbay et al 2002, Jabbour & Critchley 2001) (see website).
Implantation – myometrium
Within the myometrium, hCG and progesterone induce cellular enlargement and depress the excitability of uterine muscle by decreasing the uptake of cellular free calcium, blocking the ability of oestrogens to stimulate α-adrenergic receptors and downregulating myometrial gap junctions (Ambrus & Rao 1994, Phillips et al 2005, Ticconi et al 2007). Rising levels of progesterone act centrally to depress oxytocin neuronal activity, which is likely to cause a decline in uterine peristalsis during implantation (Rodway & Rao 1995).
Formation of the cytotrophoblast shell and gestational SAC
During the first trimester, STs actively take up secretions from the surrounding endometrial glands and contribute to gaseous and waste exchange (Ellery et al 2009). Throughout pregnancy, they are the major source of hormones, receptors, growth factors and regulatory enzymes (Burton et al 2007, Ferretti et al 2007, Habayeb et al 2008, Islami et al 2003, Jauniaux & Gulbis 2000, Tarrade et al 2001). These include hCG, human placental growth hormone (hPGH), prolactin, atrial natriuretic peptide (ANP), leptin, oestrogens, progesterone, and a growing list of regulatory glycoproteins including GnRH, corticotrophin-releasing hormone (CRH) and thyrotrophin-releasing hormone (TRH) (Cootauco et al 2008, Douglas 2010, Ferretti et al 2007, Pasqualini 2005).
As trophoblast differentiation proceeds, the ICM first subdivides into primary endoderm and ectoderm cell groups, both of which contribute to embryonic and extra-embryonic tissues (see Fig. 29.5). During the second week of life, regulatory cells within the ectoderm establish a defining organizational structure called the primitive streak, which establishes polarity of the body axis and consequently positions all embryonic organs and extra-embryonic compartments, while the endoderm group form a major part of the yolk sac, which performs the functions of a mature placenta, during the first trimester (Downs 2008, Jones 1997).
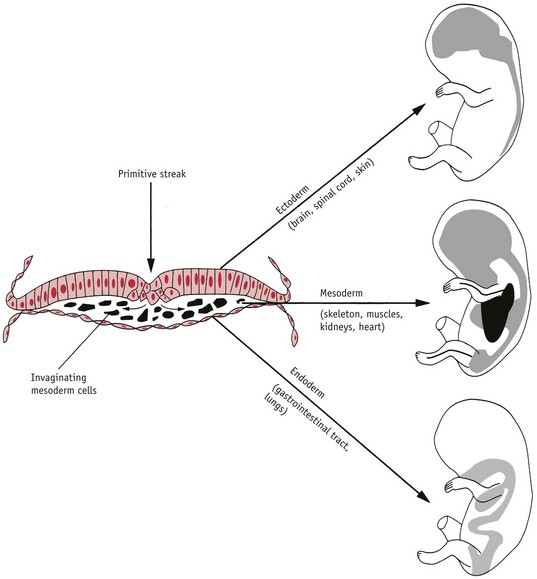
Figure 29.5 The trilaminar disc illustrating endoderm, ectoderm and mesoderm.
(Reproduced with permission from Dunstan 1990:214.)
While the cytotrophoblast shell acts as an effective barrier to maternal concentrations of oxygen, the expanding syncytiotrophoblastic mantle erodes the epithelium of surrounding endometrial glands, releasing their secretions into the extracellular matrix (Burton et al 2007). Glandular secretions enter channels forming around the cytotrophoblast shell. This activity is evident from 17 days post conception and only begins to decline when embryonic formation is complete, at the end of the first trimester (Burton et al 2007, Jauniaux & Gulbis 2000).
Histiotrophic nutrition
During the first 8–10 weeks of pregnancy, the secretory pattern of epithelial glandular cells extends that of the luteal phase, with continued glycogen secretion and a rapid rise in a number of glycoproteins (Burton et al 2001, 2007; Muller-Schottle et al 1999). These include glycodelin A, relaxin, hCG, α-tocopherol transfer protein, a powerful antioxidant, and MUC-1, a large progesterone-dependent glycoprotein (Burton et al 2002, 2007, Jauniaux et al 2004
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree