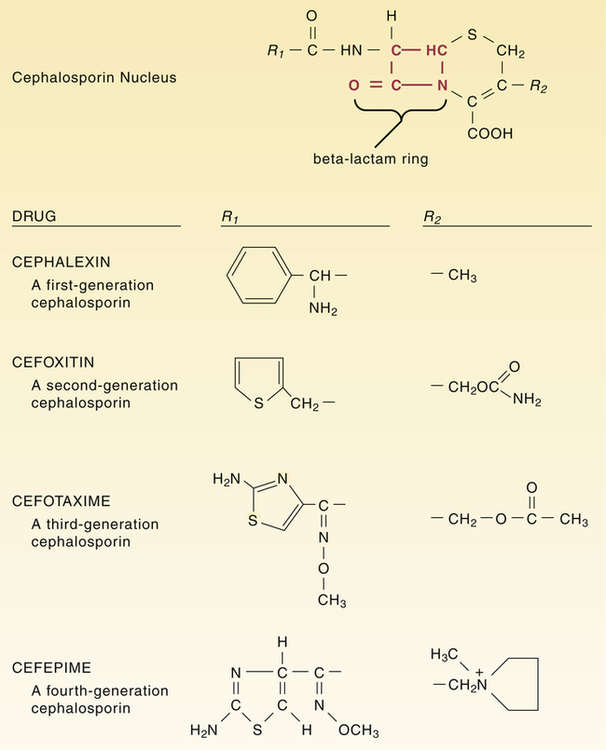
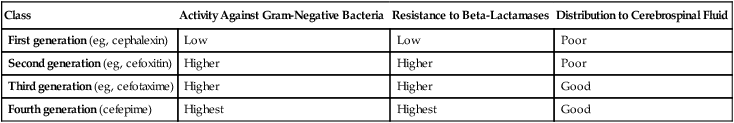
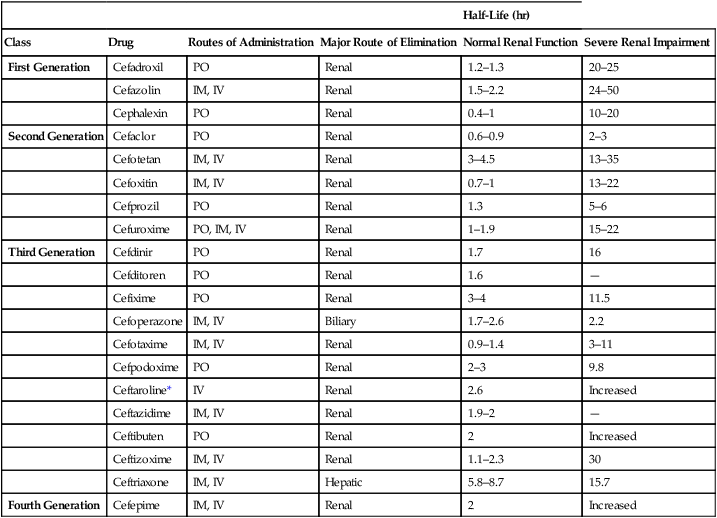
CHAPTER 85 All cephalosporins are derived from the same nucleus. As shown in Figure 85–1, this nucleus contains a beta-lactam ring fused to a second ring. The beta-lactam ring is required for antibacterial activity. Unique properties of individual cephalosporins are determined by additions made to the nucleus at the sites labeled R1 and R2. The cephalosporins can be grouped into four “generations” based on the order of their introduction to clinical use. The generations differ significantly with respect to antimicrobial spectrum and susceptibility to beta-lactamases. In general, as we progress from first-generation agents to fourth-generation agents, there is (1) increasing activity against gram-negative bacteria and anaerobes, (2) increasing resistance to destruction by beta-lactamases, and (3) increasing ability to reach the cerebrospinal fluid (CSF). These differences are summarized in Table 85–1. TABLE 85–1 Major Differences Between Cephalosporin Generations Because of poor absorption from the GI tract, many cephalosporins must be administered parenterally (IM or IV). Of the 20 cephalosporins used in the United States, only 10 can be administered by mouth (Table 85–2). Of these, only one—cefuroxime—can be administered orally and by injection. TABLE 85–2 Pharmacokinetic Properties of the Cephalosporins *Ceftaroline is classified here as a third-generation cephalosporin, because it has an antimicrobial spectrum much like that of ceftriaxone. However, ceftaroline is sometimes classified as a fifth-generation agent, because it is the only cephalosporin with activity against MRSA. Four cephalosporins—cefazolin, cefmetazole, cefoperazone, and cefotetan—can induce a state of alcohol intolerance. If a patient taking these drugs were to ingest alcohol, a disulfiram-like reaction could occur. (As discussed in Chapter 38, the disulfiram effect, which can be very dangerous, is brought on by accumulation of acetaldehyde secondary to inhibition of aldehyde dehydrogenase.) Patients using these cephalosporins must not consume alcohol in any form. • Don’t reconstitute powdered ceftriaxone with calcium-containing diluents (eg, Ringer’s solution). • Don’t mix reconstituted ceftriaxone with calcium-containing solutions. • For patients other than neonates, IV ceftriaxone and IV calcium may be administered sequentially (not concurrently) through the same line, provided the line is flushed between the infusions. • For neonates, don’t give IV ceftriaxone and IV calcium through the same line or different lines within 48 hours of each other. If the patient must receive ceftriaxone and calcium, use oral calcium or IM ceftriaxone.
Drugs that weaken the bacterial cell wall II: cephalosporins, carbapenems, vancomycin, telavancin, aztreonam, teicoplanin, and fosfomycin
Cephalosporins
Chemistry
Classification and antimicrobial spectra

Class
Activity Against Gram-Negative Bacteria
Resistance to Beta-Lactamases
Distribution to Cerebrospinal Fluid
First generation (eg, cephalexin)
Low
Low
Poor
Second generation (eg, cefoxitin)
Higher
Higher
Poor
Third generation (eg, cefotaxime)
Higher
Higher
Good
Fourth generation (cefepime)
Highest
Highest
Good
Pharmacokinetics
Absorption.

Half-Life (hr)
Class
Drug
Routes of Administration
Major Route of Elimination
Normal Renal Function
Severe Renal Impairment
First Generation
Cefadroxil
PO
Renal
1.2–1.3
20–25
Cefazolin
IM, IV
Renal
1.5–2.2
24–50
Cephalexin
PO
Renal
0.4–1
10–20
Second Generation
Cefaclor
PO
Renal
0.6–0.9
2–3
Cefotetan
IM, IV
Renal
3–4.5
13–35
Cefoxitin
IM, IV
Renal
0.7–1
13–22
Cefprozil
PO
Renal
1.3
5–6
Cefuroxime
PO, IM, IV
Renal
1–1.9
15–22
Third Generation
Cefdinir
PO
Renal
1.7
16
Cefditoren
PO
Renal
1.6
—
Cefixime
PO
Renal
3–4
11.5
Cefoperazone
IM, IV
Biliary
1.7–2.6
2.2
Cefotaxime
IM, IV
Renal
0.9–1.4
3–11
Cefpodoxime
PO
Renal
2–3
9.8
Ceftaroline*
IV
Renal
2.6
Increased
Ceftazidime
IM, IV
Renal
1.9–2
—
Ceftibuten
PO
Renal
2
Increased
Ceftizoxime
IM, IV
Renal
1.1–2.3
30
Ceftriaxone
IM, IV
Hepatic
5.8–8.7
15.7
Fourth Generation
Cefepime
IM, IV
Renal
2
Increased
Drug interactions
Alcohol.
Calcium and ceftriaxone.