CHAPTER 59 The hypothalamus and pituitary are intimately related both anatomically and functionally. Working together, these structures help regulate practically all bodily processes. To achieve their widespread effects, the hypothalamus and pituitary employ at least 15 hormones and regulatory factors (Fig. 59–1). As you can imagine, the endocrinology of these structures is exceedingly complex. However, rather than discussing all relevant information in depth, we will focus on just three agents: growth hormone (GH), antidiuretic hormone (ADH), and prolactin. Additional hypothalamic and pituitary hormones of therapeutic interest are considered briefly here and discussed further in other chapters. The pituitary sits in a depression in the skull located just below the third ventricle of the brain; the hypothalamus is located immediately above (see Fig. 59–1). The pituitary has two divisions: the anterior pituitary (or adenohypophysis) and posterior pituitary (or neurohypophysis). Both divisions are under hypothalamic control. As indicated in Figure 59–1, the hypothalamus communicates with the anterior pituitary by way of release-regulating factors delivered through a system of portal blood vessels. In contrast, communication with the posterior pituitary is neuronal. • Growth hormone (GH) stimulates growth in practically all tissues and organs. • Corticotropin (adrenocorticotropic hormone [ACTH]) acts on the adrenal cortex to promote synthesis and release of adrenocortical hormones. • Thyrotropin (thyroid-stimulating hormone [TSH]) acts on the thyroid gland to promote synthesis and release of thyroid hormones. • Follicle-stimulating hormone (FSH) acts on the ovaries to promote follicular growth and development, and acts on the testes to promote spermatogenesis. • Luteinizing hormone (LH) acts on the ovaries to promote ovulation and development of the corpus luteum, and acts on the testes to promote androgen production. Although oxytocin and ADH are considered hormones of the posterior pituitary, these agents are actually synthesized in the hypothalamus. The cells that make oxytocin and ADH are called neurosecretory cells. As indicated in Figure 59–1, these cells originate in the hypothalamus and project their axons to the posterior pituitary. Oxytocin and ADH are produced within the bodies of these cells and then undergo transport down axons to the axon terminals for storage. When appropriate stimuli impinge upon the bodies of the neurosecretory cells, impulses are sent down the axon, causing hormone release. The hypothalamus has the primary responsibility for regulating the release of hormones from the anterior pituitary. To accomplish this, the hypothalamus employs seven different release-regulating factors (see Fig. 59–1). Most of these factors stimulate the release of anterior pituitary hormones. However, two of these factors inhibit hormone release. As indicated in Figure 59–1, the hypothalamic release-regulating factors are delivered to the anterior pituitary via portal blood vessels. Although the hypothalamic releasing factors are of extreme physiologic importance, only five of them—growth hormone–releasing hormone, thyrotropin-releasing hormone, gonadotropin-releasing hormone, corticotropin-releasing hormone, and somatostatin—have clinical applications. These are the only hypothalamic release-regulating factors discussed in this chapter. With few exceptions, the release of hypothalamic and anterior pituitary hormones is regulated by a negative feedback loop, as illustrated in Figure 59–2. In this example, the loop begins with the secretion of releasing-factor X from the hypothalamus. Factor X then acts on the anterior pituitary to stimulate release of hormone A. Hormone A then acts on its target gland to promote release of hormone B. Hormone B has two actions: (1) it produces its designated biologic effects and (2) it acts on the hypothalamus and pituitary to inhibit further release of factor X and hormone A. This feedback inhibition of the hypothalamus and pituitary suppresses further release of hormone B itself, thereby keeping levels of hormone B within an appropriate range. The factors that regulate GH release are summarized in Figure 59–3. As indicated, the hypothalamus first releases growth hormone–releasing hormone (GH-RH), which stimulates release of GH from the pituitary. Growth hormone then acts on the liver and other tissues to cause release of insulin-like growth factor-1 (IGF-1). IGF-1 has two actions: it (1) promotes growth and (2) acts on the hypothalamus and pituitary to suppress release of GH-RH and GH, thereby completing a negative feedback loop. One additional hormone—somatostatin—helps regulate GH release. As shown in Figure 59–3, somatostatin, which is produced in the hypothalamus, acts on the pituitary to inhibit GH release. Growth hormone is essential for normal growth of children, and hence GH deficiency results in short stature. Growth is retarded to an equal extent in all parts of the body, and hence the child, although short, has normal proportions. Mental function is not impaired. The only treatment for GH deficiency is replacement therapy with human GH itself (see below under Therapeutic Uses). Drugs are generally reserved for patients with large tumors or residual disease despite tumor excision and/or radiation therapy. Three drugs are available: octreotide [Sandostatin, Sandostatin LAR Depot], lanreotide [Somatuline Depot], and pegvisomant [Somavert]. The pharmacology of these agents is discussed later under Drugs for Acromegaly.
Drugs related to hypothalamic and pituitary function
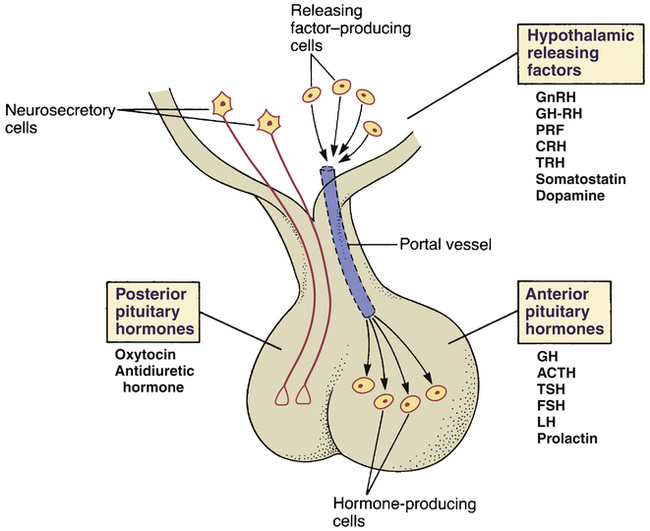
 Hormones and releasing factors of the hypothalamus and pituitary.
Hormones and releasing factors of the hypothalamus and pituitary.
Hypothalamic releasing factors: GnRH = gonadotropin-releasing hormone, GH-RH = growth hormone–releasing hormone, PRF = prolactin-releasing factor, CRH = corticotropin-releasing hormone, TRH = thyrotropin-releasing hormone.
Anterior pituitary hormones: GH = growth hormone, ACTH = adrenocorticotropic hormone (corticotropin), TSH = thyroid-stimulating hormone, FSH = follicle-stimulating hormone, LH = luteinizing hormone.
Overview of hypothalamic and pituitary endocrinology
Anatomic considerations
Hormones of the anterior pituitary
Hormones of the posterior pituitary
Hypothalamic release-regulating factors
Feedback regulation of the hypothalamus and anterior pituitary
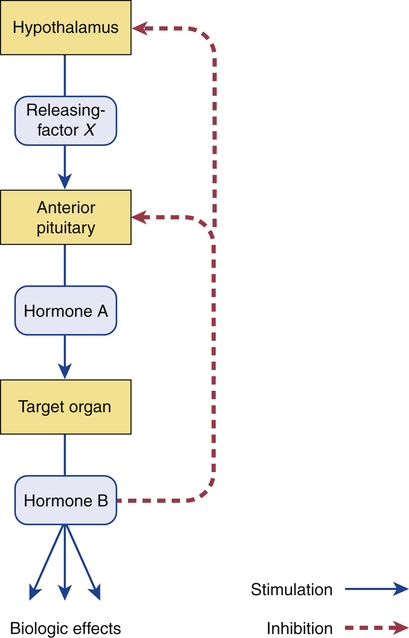
 Negative feedback regulation of the hypothalamus and anterior pituitary.
Negative feedback regulation of the hypothalamus and anterior pituitary.
The feedback loop works as follows: Factor X stimulates the pituitary to release hormone A, which stimulates its target organ, causing release of hormone B. Hormone B then acts on the hypothalamus and pituitary to suppress further release of factor X and hormone A, thereby suppressing further release of hormone B itself.
Growth hormone
Physiology
Regulation of release
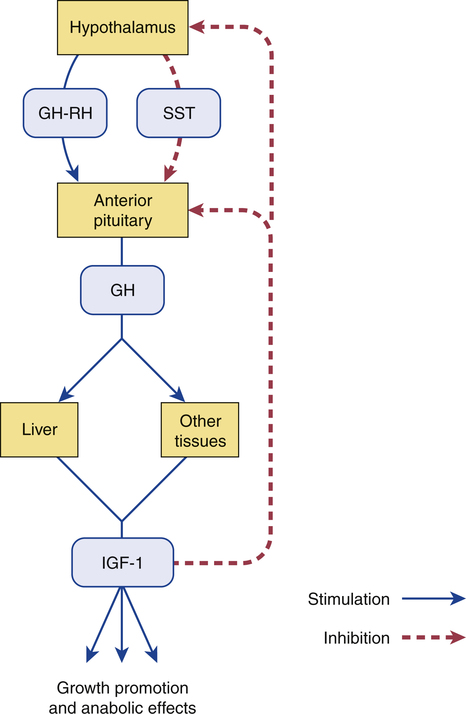
 Regulation of growth hormone release.
Regulation of growth hormone release.
(GH = growth hormone, GH-RH = growth hormone–releasing hormone, IGF-1 = insulin-like growth factor-1; SST = somatostatin.)
Pathophysiology
Growth hormone deficiency
Pediatric.
Growth hormone excess
Treatment overview.




