CHAPTER 60 Glucocorticoids are so named because they increase the availability of glucose. Of the several glucocorticoids produced by the adrenal cortex, cortisol is the most important. The structural formula of cortisol is shown in Figure 60–1. When considering the glucocorticoids, we need to distinguish between physiologic effects and pharmacologic effects. Physiologic effects occur at low levels of glucocorticoids (ie, the levels produced by release of glucocorticoids from healthy adrenals, or by administering glucocorticoids in low doses). Pharmacologic effects occur at high levels of glucocorticoids. These levels are achieved when glucocorticoids are administered in the large doses required to treat disorders unrelated to adrenocortical function (eg, allergic reactions, asthma, inflammation, cancer). Pharmacologic levels can also be reached when production of endogenous glucocorticoids is excessive, as occurs in Cushing’s disease. In this chapter, we focus on the physiologic role of glucocorticoids. The use of high-dose glucocorticoids for nonendocrine purposes is discussed in Chapter 72. Glucocorticoid synthesis and release are regulated by a negative feedback loop (Fig. 60–2). The loop begins with release of corticotropin-releasing hormone (CRH) from the hypothalamus. CRH acts on the anterior pituitary to promote release of adrenocorticotropic hormone (ACTH), which stimulates the zona fasciculata of the adrenal cortex, causing synthesis and release of cortisol and other glucocorticoids. Following release, cortisol acts in two ways: (1) it promotes its designated biologic effects and (2) it acts on the hypothalamus and pituitary to suppress further release of CRH and ACTH. Hence, as cortisol levels rise, they act to suppress further stimulation of glucocorticoid production, thereby keeping glucocorticoid levels within an appropriate range. The hypothalamic-pituitary-adrenal system is activated by signals from the CNS. These signals turn the system on by causing the hypothalamus to release CRH. As indicated in Figure 60–2, two modes of activation are involved. One provides a basal level of stimulation; the other increases stimulation at times of stress. Basal stimulation follows a circadian rhythm: Cortisol levels are lowest near bedtime, rise during sleep, reach a peak just before waking, and then decline through the day. (Note that this cycle is linked to one’s sleep pattern, and not to the clock. Hence, for some people, cortisol may peak in the morning, and for others it may peak in the afternoon or evening, depending on when they normally sleep.) When stress occurs, glucocorticoid production goes up. Stressful events that can activate the loop include injury, infection, and surgery. The signals generated by stress produce intense stimulation of the hypothalamus. The resultant release of CRH and ACTH can cause plasma levels of cortisol to increase 10-fold. Because stress is such a powerful stimulus, it overrides feedback inhibition by cortisol. Secretion of aldosterone is regulated by the renin-angiotensin-aldosterone system (RAAS), not by ACTH. The mechanisms by which the RAAS regulates aldosterone are discussed in Chapter 44. It is important to note that, because aldosterone is not regulated by ACTH, conditions that alter secretion of ACTH do not alter secretion of aldosterone. Signs and symptoms of Cushing’s syndrome result from excess levels of circulating glucocorticoids. Principal causes are (1) hypersecretion of ACTH by pituitary adenomas (Cushing’s disease), (2) hypersecretion of glucocorticoids by adrenal adenomas and carcinomas, and (3) administering glucocorticoids in the large doses used to treat arthritis and other nonendocrine disorders (see Chapter 72).
Drugs for disorders of the adrenal cortex
Physiology of the adrenocortical hormones
Glucocorticoids
Regulation of synthesis and secretion
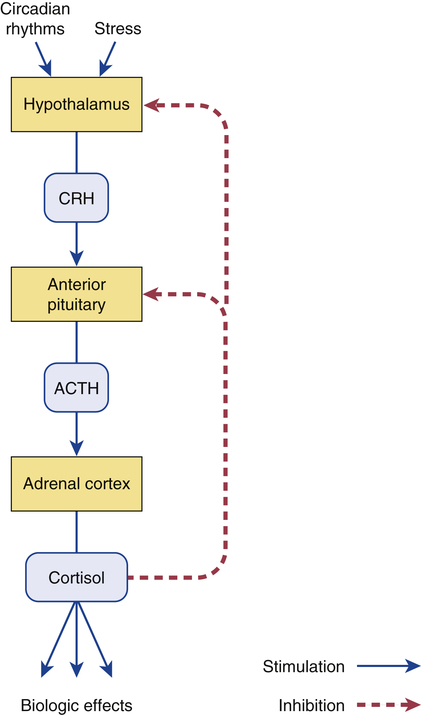
 Negative feedback regulation of glucocorticoid synthesis and secretion.
Negative feedback regulation of glucocorticoid synthesis and secretion.
(ACTH = adrenocorticotropic hormone, CRH = corticotropin-releasing hormone.)
Mineralocorticoids
Control of secretion.
Pathophysiology of the adrenocortical hormones
Adrenal hormone excess
Cushing’s syndrome
Causes.
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Drugs for disorders of the adrenal cortex
Only gold members can continue reading. Log In or Register to continue
Get Clinical Tree app for offline access