Discuss the role of prostaglandins in the etiology of pain, fever, and inflammation.
 Identify the major manifestations of fever and inflammation.
Identify the major manifestations of fever and inflammation.
 Understand the pathophysiology of osteoarthritis.
Understand the pathophysiology of osteoarthritis.
 Understand the pathophysiology of gout.
Understand the pathophysiology of gout.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the salicylates.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the salicylates.
 Identify the action, use, adverse effects, contraindications, and nursing implications for acetaminophen.
Identify the action, use, adverse effects, contraindications, and nursing implications for acetaminophen.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the propionic acid derivatives.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the propionic acid derivatives.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the oxicam derivatives.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the oxicam derivatives.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications of the acetic acid derivatives.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications of the acetic acid derivatives.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications of the selective COX-2 inhibitors.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications of the selective COX-2 inhibitors.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the mitotic agents.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the mitotic agents.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for uricosuric medications.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for uricosuric medications.
 Know how to implement the nursing process in the care of patients undergoing drug therapy for pain, fever, and inflammation.
Know how to implement the nursing process in the care of patients undergoing drug therapy for pain, fever, and inflammation.
Clinical Application Case Study
Audrey Mason is a 72-year-old retired physical education teacher and housewife, who is being seen by her primary care provider for bilateral hip and knee pain. She has been taking a minimum of eight 325 mg aspirins per day without pain relief. Her physician orders x-rays of her hips and knees, which reveal osteoarthritis in both joints. She begins taking ibuprofen (Motrin) 600 mg three times per day. In addition, she receives a diagnosis of degenerative joint disease related to osteoarthritis and a referral to an orthopedic surgeon. The surgeon schedules Mrs. Mason for a right total knee replacement in 1 month. Because she is 40 pounds overweight, the surgeon places her on a calorie reduction diet.
KEY TERMS
Antiprostaglandin: drug that inhibits the synthesis of prostaglandins
Antipyretic: drug that has the ability to lower body temperature
Arachidonic acid: phospholipid released in the cell membrane in response to cellular injury Cyclooxygenase: enzyme that produces prostaglandins from arachidonic acids
Hyperuricemia: elevated levels of uric acid in the blood resulting from accelerated generation of uric acid through purine metabolism or impaired renal excretion of uric acid
Nonsteroidal anti-inflammatory drug (NSAID): medication that inhibits the synthesis of prostaglandins; used to prevent and treat mild to moderate pain and inflammation
Prostaglandin: chemical mediator found in most body tissues; helps regulate many cell functions and participate in the inflammatory response as well as initiate uterine contractions in labor
Pyrogen: fever-producing agent
Reye’s syndrome: potentially fatal disease characterized by encephalopathy and fatty liver accumulations; associated with the use of aspirin and NSAIDs after viral infections such as chickenpox or influenza in children and adolescents
Salicylism: toxic effects of a salicylate drug; may occur with an acute overdose or with chronic use of therapeutic doses, especially the higher doses take for anti-inflammatory effects
Tophi: deposits of uric acid crystals in the joints, kidneys, and soft tissues
Uricosuric: drug that increases urinary excretion of uric acid
Introduction
This chapter provides an introduction to the pharmacological care of the patient who is experiencing pain, fever, or inflammation. The pharmacological agents administered for inflammation can also diminish fever and relieve pain. Acetaminophen also decreases fever and relieve pain, but it does not reduce inflammation. Other topics of discussion will be osteoarthritis and gout, which allows the nurse to apply the knowledge of disease that produces inflammation and the related administration of anti-inflammatory agents to reduce pain. The drugs discussed in this chapter include aspirin (acetylsalicylic acid), acetaminophen, and the nonsteroidal antiinflammatory drugs (NSAIDs), as well as those drugs used to prevent or treat gout.
It is important to note that aspirin, acetaminophen, and NSAIDs can also be called antiprostaglandins because they inhibit the synthesis of prostaglandins. Prostaglandins are chemical mediators found in most body tissues; they help regulate many cell functions and participate in the inflammatory response. They are formed when cellular injury occurs and phospholipids in cell membranes respond by releasing arachidonic acid. Cyclooxygenase (COX) enzymes then metabolize arachidonic acid to produce prostaglandins, which act briefly in the area where they are produced and are then inactivated. The enzyme COX-1 is normally synthesized continuously and is present in all tissues and cell types, especially in platelets and endothelial cells as well as in the gastrointestinal (GI) tract and the kidneys. Prostaglandins produced by COX-1 are important in numerous homeostatic functions and have protective effects on the stomach and kidneys. In the stomach, prostaglandins decrease gastric acid secretion, increase mucus secretion, and regulate blood circulation. In the kidneys, they help maintain adequate blood flow and function. In the cardiovascular system, they help regulate vascular tone (i.e., vasoconstriction and vasodilation) and platelet function. Drug-induced inhibition of these prostaglandins results in the adverse effects associated with aspirin and related nonselective NSAIDs, especially gastric irritation, ulceration, and bleeding. Inhibition of COX-1 activity in platelets may be more responsible for GI bleeding than inhibition of COX-1 activity in gastric mucosa.
COX-2 is also normally present in several tissues (e.g., brain, bone, kidneys, GI tract, female reproductive system). However, it is thought to occur in small amounts or to be inactive until stimulated by pain and inflammation. In inflamed tissues, COX-2 is induced by inflammatory chemical mediators such as interleukin-1 and tumor necrosis factor-alpha. In the GI tract, trauma and Helicobacter pylori infection, a common cause of peptic ulcer disease, also induce COX-2. Overall, prostaglandins produced by COX-2 are associated with pain and other signs of inflammation. Inhibition of COX-2 results in the therapeutic effects of analgesia and anti-inflammatory activity. The COX-2 inhibitor drugs are NSAIDs designed to selectively inhibit COX-2 and relieve pain and inflammation with fewer adverse effects than those that inhibit both COX-1 and COX-2, especially stomach damage. However, with long-term use, adverse effects still occur in the GI, renal, and cardiovascular systems.
As indicated in Table 14.1 and Figure 14.1, prostaglandins exert various and opposing effects in different body tissues.
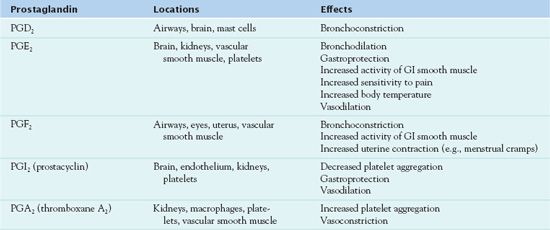
GI, gastrointestinal.
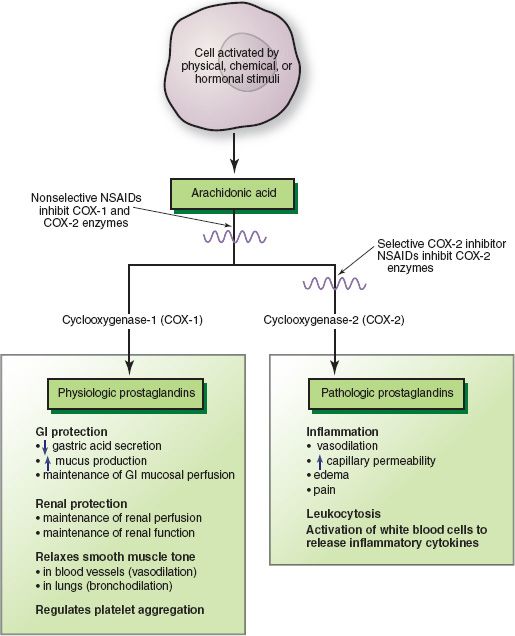
Figure 14.1 Metabolic pathways for arachidonic acid result in production of physiologic and pathologic (i.e., inflammatory prostaglandins). Nonselective and selective nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit production of prostaglandins by inhibiting steps in the arachidonic acid pathway. GI, gastrointestinal.
Overview of Pain, Fever, and Inflammation
To adequately understand the administration of aspirin, acetaminophen, and NSAIDs, it is important to understand the role prostaglandins play in mediating pain, fever, and inflammation.
Pain
Pain is the sensation of discomfort, hurt, or distress. It is a common human ailment and may occur with tissue injury and inflammation. Prostaglandins sensitize pain receptors and increase the pain associated with other chemical mediators of inflammation and immunity, such as bradykinin, histamine, and leukotrienes (Box 14.1).
BOX 14.1 Chemical Mediators of Inflammation and Immunity
Bradykinin is a kinin in body fluids that becomes physiologically active with tissue injury. When tissue cells are damaged, white blood cells (WBCs) increase in the area and ingest damaged cells to remove them from the area. When the WBCs die, they release enzymes that activate kinins. The activated kinins increase and prolong the vasodilation and increased vascular permeability caused by histamine. They also cause pain by stimulating nerve endings for pain in the area. Thus, bradykinin may aggravate and prolong the erythema, heat, and pain of local inflammatory reactions. It also increases mucous gland secretion.
Complement is a group of plasma proteins essential to normal inflammatory and immunologic processes. More specifically, complement destroys cell membranes of body cells (e.g., red blood cells, lymphocytes, platelets) and pathogenic microorganisms (e.g., bacteria, viruses). The system is initiated by an antigen-antibody reaction or by tissue injury. Components of the system (called C1 through C9) are activated in a cascade type of reaction in which each component becomes a proteolytic enzyme that splits the next component in the series. Activation yields products with profound inflammatory effects. C3a and C5a, also called anaphylatoxins, act mainly by liberating histamine from mast cells and platelets, and their effects are therefore similar to those of histamine. C3a causes or increases smooth muscle contraction, vasodilation, vascular permeability, degranulation of mast cells and basophils, and secretion of lysosomal enzymes by leukocytes. C5a performs the same functions as C3a and also promotes movement of WBCs into the injured area (chemotaxis). In addition, it activates the lipoxygenase pathway of arachidonic acid metabolism in neutrophils and macrophages, thereby inducing formation of leukotrienes and other substances that increase vascular permeability and chemotaxis.
In the immune response, the complement system breaks down antigen-antibody complexes, especially those in which the antigen is a microbial agent. It enables the body to produce inflammation and localize an infective agent. More specific reactions include increased vascular permeability, chemotaxis, and opsonization (coating a microbe or other antigen so it can be more readily phagocytized).
Cytokines may act on the cells that produce them, on surrounding cells, or on distant cells if sufficient amounts reach the bloodstream. Thus, cytokines act locally and systemically to produce inflammatory and immune responses, including increased vascular permeability and chemotaxis of macrophages, neutrophils, and basophils. Two major types of cytokines are interleukins (produced by leukocytes) and interferons (produced by T lymphocytes or fibroblasts). Interleukin-1 (IL-1) mediates several inflammatory responses, including fever; IL-2 (also called T-cell growth factor) is required for the growth and function of T lymphocytes. Interferons are cytokines that protect nearby cells from invasion by intracellular microorganisms, such as viruses and rickettsiae. They also limit the growth of some cancer cells.
Histamine is formed (from the amino acid histidine) and stored in most body tissue, with high concentrations in mast cells, basophils, and platelets. Mast cells, which are abundant in skin and connective tissue, release histamine into the vascular system in response to stimuli (e.g., antigen-antibody reaction, tissue injury, and some drugs). After it is released, histamine is highly vasoactive, causing vasodilation (increasing blood flow to the area and producing hypotension) and increasing permeability of capillaries and venules (producing edema). Other effects include contracting smooth muscles in the bronchi (producing bronchoconstriction and respiratory distress), gastrointestinal (GI) tract, and uterus; stimulating salivary, gastric, bronchial, and intestinal secretions; stimulating sensory nerve endings to cause pain and itching; and stimulating movement of eosinophils into injured tissue. Histamine is the first chemical mediator released in the inflammatory response and immediate hypersensitivity reactions (anaphylaxis).
When histamine is released from mast cells and basophils, it diffuses rapidly into other tissues. It then acts on target tissues through both histamine-1 (H1) and histamine-2 (H2) receptors. H1 receptors are located mainly on smooth muscle cells in blood vessels and the respiratory and GI tracts. When histamine binds with these receptors, resulting events include contraction of smooth muscle, increased vascular permeability, production of nasal mucus, stimulation of sensory nerves, pruritus, and dilation of capillaries in the ski n. H2 receptors are also located in the airways, GI tract, and other tissues. When histamine binds to these receptors, there is increased secretion of gastric acid by parietal cells in the stomach mucosal lining, increased mucus secretion and bronchodilation in the airways, contraction of esophageal muscles, tachycardia, inhibition of lymphocyte function, and degranulation of basophils (with additional release of histamine and other mediators) in the bloodstream. In allergic reactions, both types of receptors mediate hypotension (in anaphylaxis), skin flushing, and headache. The peak effects of histamine occur within 1 to 2 minutes of its release and may last as long as 10 minutes, after which it is inactivated by histaminase (produced by eosinophils) or N-methyltransferase.
Leukotrienes, like prostaglandins, are derived from arachidonic acid metabolism. Leukotrienes, identified as LTB4, LTC4, LTD4, and LTE4, mediate inflammation and immune responses. LTB4 plays a role in chemotaxis, mediating the aggregation of leukocytes at sites of injury. LTC4, LTD4, and LTE4 produce smooth muscle contractility, bronchospasm, and increased vascular permeability.
Nitric oxide (NO) is synthesized by a variety of cells by the enzyme NO synthase from the amino acid arginine. It readily diffuses across cell membranes, where it reacts with a wide variety of molecules and is inactivated. NO inhibits aggregation of platelets, preventing formation of blood clots. NO relaxes smooth muscles in blood vessels, producing vasodilation. It inhibits inflammation in the walls of blood vessels. NO also plays a role in protecting against invading microbes. Helper T lymphocytes, active in the inflammatory response, secrete NO. NO also enhances the killing of phagocytized microbes within the lysosomes of cells.
Platelet-activating factor (PAF), like prostaglandins and leukotrienes, is derived from arachidonic acid metabolism and has multiple inflammatory activities. It is produced by mast cells, neutrophils, monocytes, and platelets. Because these cells are widely distributed, PAF effects can occur in virtually every organ and tissue. Besides causing platelet aggregation, PAF activates neutrophils, attracts eosinophils, increases vascular permeability, causes vasodilation, and causes IL-1 and tumor necrosis factor-alpha (TNF-alpha) to be released. PAF, IL-1, and TNF-alpha can induce each other’s release.
Fever
Fever is an elevation of body temperature above the normal range. Body temperature is controlled by a regulating center in the hypothalamus. Normally, there is a balance between heat production and heat loss so that a constant body temperature is maintained. When there is excessive heat production, mechanisms to increase heat loss are activated. As a result, blood vessels dilate, more blood flows through the skin, sweating occurs, and body temperature usually stays within normal range.
Fever occurs when the set point of the hypothalamus is raised in response to the presence of pyrogens (fever-producing agents). Endogenous pyrogens include cytokines such as interleukin-1, interleukin-6, and tumor necrosis factor (see Box 14.1). Exogenous pyrogens include bacteria and their toxins or other by-products. The upward adjustment of the hypothalamic set point in response to the presence of a pyrogen is mediated by prostaglandin E2 (see Table 14.1). The body responds to the higher hypothalamic set point by vasoconstriction of blood vessels and shivering, raising the core body temperature to the higher set point. Fever may accompany conditions such as dehydration, inflammation, infectious processes, some drug use, brain injury, or diseases involving the hypothalamus.
Inflammation
Inflammation is the normal body response to tissue damage from any source, and it may occur in any tissue or organ. It is an attempt by the body to remove the damaging agent and repair the damaged tissue. The signs and symptoms of inflammation are the work of a variety of chemical mediators (see Box 14.1). Prostaglandin E2 and others induce inflammation and also enhance the effects of other mediators of the inflammatory response. Local manifestations are redness, heat, edema, and pain. Redness and heat result from vasodilation and increased blood supply. Edema results from leakage of blood plasma into the area. Pain occurs when pain receptors on nerve endings are stimulated by heat, edema, and pressure; chemicals released by the damaged cells; and prostaglandins. Systemic manifestations include leukocytosis, increased erythrocyte sedimentation rate, fever, headache, loss of appetite, lethargy or malaise, and weakness. Both local and systemic manifestations vary according to the cause and extent of tissue damage. In addition, inflammation may be acute or chronic. See Chapter 13 for more information.
Inflammation may be a component of virtually any illness. Inflammatory conditions affecting organs or systems are often named by adding the suffix “itis” to the involved organ or system (e.g., hepatitis). Current research findings suggest that inflammation may be important in the pathology of disorders (not previously identified as inflammatory conditions) such as heart disease and Alzheimer’s disease. Anti-inflammatory drugs are indicated when the inflammatory response is inappropriate, abnormal or persistent, or destroys tissue.
Table 14.2 lists the medications administered in the reduction of pain, fever, and inflammation.
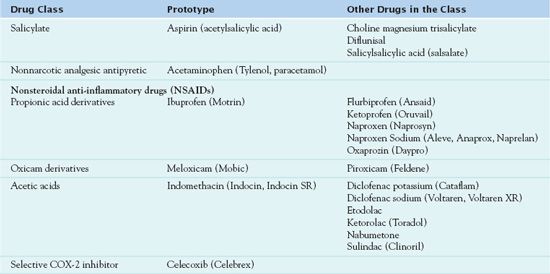
COX-2, cyclooxygenase-2.
Specific Conditions
OSTEOARTHRITIS
Osteoarthritis produces inflammation and degeneration of joints. To adequately understand the pharmacologic treatment of osteoarthritis, it is important to understand the etiology, pathophysiology, and clinical manifestations of the disease.
Etiology
Aging plays a significant role in the destruction of joints. The degeneration of the articular cartilage begins at approximately 30 years of age and peaks between 50 and 60 years. Primary osteoarthritis occurs without a history of an injury or disease. Secondary osteoarthritis results from a previous injury or the presence of an inflammatory process. Other factors that contribute to the development of osteoarthritis include obesity, repetitive use of a joint, and congenital predisposition.
Pathophysiology
The articular cartilage is the smooth weight-bearing surface at the ends of long bones. The main function of cartilage is to absorb shock and decrease friction during movement and weight-bearing activities. The cartilage transmits the load down to the subchondral bone, dissipating the mechanical stress at the joint. Along with the synovial fluid in the joint, the articular cartilage reduces friction when the joint moves. Osteoarthritis is the degradation of the cartilage, bone, and synovium. Repetitive movement of a joint causes the articular cartilage to be worn down, leading to joint failure. Chondrocytes develop in the joint with the release of enzymes, causing the joint to degenerate. Progressively the articular cartilage is lost, and inflammation develops in the synovial fluid.
Clinical Manifestations
Osteoarthritis produces joint pain, stiffness, and instability, possibly with some degree of immobility. The joints commonly affected by osteoarthritis are the carpometacarpal joint (the distal joint of the hand), metatarsophalangeal joint of the feet, knees, hips, and cervical or lumbar vertebrae. The pain experienced by the patient is related to the inflammation of the synovium. As the synovial fluid increases, the joint capsule becomes stretched and causes irritation to the nerve endings of the periosteum. Joint stiffness is most common on arising, especially on awaking in the morning, and decreases with movement. An impaired joint can limit mobility and cause structural changes.
Drug Therapy
Medications used in the treatment of osteoarthritis include aspirin, acetaminophen, and NSAIDs (see Table 14.2). As previously mentioned, all of these medications produce an analgesic effect and reduce fever. However, only aspirin and the NSAIDs reduce inflammation. As the medications are discussed, their properties will be explained in detail, identifying their use and implications for administration.
Clinical Application 14-1
 In an education session with Mrs. Mason, the nurse employed by Mrs. Mason’s insurance provider is instructing her about the pathophysiology of osteoarthritis. What information should the nurse provide?
In an education session with Mrs. Mason, the nurse employed by Mrs. Mason’s insurance provider is instructing her about the pathophysiology of osteoarthritis. What information should the nurse provide?
 What is the rationale for placing Mrs. Mason on a calorie reduction diet?
What is the rationale for placing Mrs. Mason on a calorie reduction diet?
NCLEX Success
1. A man arrives in the emergency department with a swollen right ankle from a fall. He is complaining of pain and limited mobility. What factor is contributing to the pain in the right ankle?
A. blocking of cyclooxygenase-1 (COX-1)
B. blocking of COX-1 and COX-2
C. release of prostaglandin E2
D. release of cytochrome P450
2. A home care nurse is visiting an 88-year-old man, who is taking acetaminophen for arthritic pain in his knees. Which of the following patient teaching statements is most appropriate to implement?
A. “Acetaminophen will only relieve pain but not the inflammation from arthritis.”
B. “Acetaminophen is appropriate for the treatment of inflammation from arthritis.”
C. “Your primary health care provider should consider a prescription of Vicodin (acetaminophen/hydrocodone).”
D. “The acetaminophen should be administered on an empty stomach.”
3. A patient is admitted to the emergency department with dehydration. Which of the following assessments of the patient’s vital signs does the nurse expect to assess?
A. elevated blood pressure
B. diminished pulse
C. diminished respirations
D. elevated temperature
GOUT
Gout is an arthritic condition characterized by an overproduction of uric acid or an inability to excrete uric acid, resulting in hyperuricemia. Uric acid is a by-product of purine metabolism. Hyperuricemia occurs when the serum uric acid level exceeds 6.8 mg/dL, the saturation point at which urate crystallizes in biological fluids at normal body temperature (Hilaire & Wozniak, 2010). According to Schub & Pravikoff (2011), there are three stages of gout. Acute gouty arthritis or gouty attack, the first stage, is characterized by hyperuricemia, pain, and swelling of the joints. The pain usually begins at night and persists for 10 days. The most commonly affected joint is the great toe. Intercritical gout, the second stage, is characterized by a symptom-free period of several years followed by the recurrence of symptoms. Chronic tophaceous gout, the third stage, is characterized by the presence of solid deposits of urate crystals, known as tophi, in the joints and elsewhere (Fig. 14.2). In the kidneys, urate deposits may form renal calculi or cause other damage. After the first attack of acute gouty arthritis, up to 10 years may pass before permanent damage to the joints and kidneys occurs.
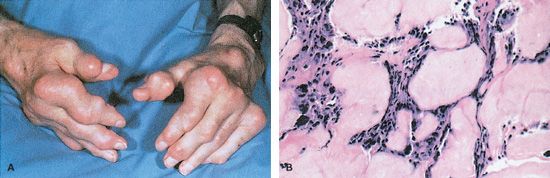
Figure 14.2 Gouty tophi projections.
The treatment of gout involves the administration of NSAIDs and corticosteroids (see Chap. 15) to reduce inflammation as well as uricosuric agents to increase the elimination of uric acid. Table 14.3 lists the antigout medications and uricosuric agents. These drugs will be discussed later in this chapter.
Salicylates
The salicylates, of which  aspirin is the prototype, relieve pain by acting both centrally and peripherally to block the transmission of pain impulses. They act peripherally to prevent the sensation of pain receptors to various chemical substances releases by damaged cells. These antipyretic agents also reduce fever by acting on the hypothalamus to decrease its response to pyrogens and resetting the body temperature at a lower level. In addition, these drugs diminish inflammation by preventing prostaglandins from increasing the pain and edema produced by other substances released by damaged cells.
aspirin is the prototype, relieve pain by acting both centrally and peripherally to block the transmission of pain impulses. They act peripherally to prevent the sensation of pain receptors to various chemical substances releases by damaged cells. These antipyretic agents also reduce fever by acting on the hypothalamus to decrease its response to pyrogens and resetting the body temperature at a lower level. In addition, these drugs diminish inflammation by preventing prostaglandins from increasing the pain and edema produced by other substances released by damaged cells.
Aspirin and other salicylates also have the ability to suppress platelet aggregation. Low-dose aspirin is indicated for patients who have experienced an ischemic stroke, transient ischemic attack, angina, and acute myocardial infarction (or any myocardial infarction), reducing the risk of death and/or a recurrent event (level A recommendations). This indication stems from its antiplatelet activity and resultant effects on blood coagulation (i.e., decreased clot formation). Low-dose aspirin is also be used for primary prevention of myocardial infarction or stroke in healthy adults.
Pharmacokinetics
Aspirin is administered orally or rectally with an onset of action of 5 to 30 minutes orally and 1 to 2 hours rectally. The oral preparation peaks in 15 to 120 minutes, and the duration of action is 3 to 6 hours. The rectal preparation peaks in 4 to 5 hours, and the duration of action is 6 to 8 hours. The drug is metabolized in the liver and has a half-life of 15 minutes to 12 hours. Excretion takes place in the urine. The drug crosses the placenta and enters the breast milk.
Action
The ability of aspirin to inhibit prostaglandins produces the inflammatory effects needed for analgesia and antirheumatic effects. Its antipyretic effects are less well understood. Authorities believe that the drug acts on the thermoregulatory center of the hypothalamus, thus blocking the effects of the endogenous pyrogens and inhibiting the synthesis of prostaglandins. Aspirin also has antiplatelet effects. At low doses, it blocks the synthesis of thromboxane A2 to inhibit platelet aggregation; this lasts for the life of the platelet.
Use
As an analgesic agent, aspirin is used to relieve mild to moderate pain. As an antipyretic agent, it is used only in adults (see “Use in Children”). As an anti-inflammatory agent, it is used to decrease inflammation in patients with osteoarthritis, juvenile rheumatoid arthritis, and spondyloarthropathies. In addition, aspirin is used for its antiplatelet effects to reduce the risk of transient ischemic attacks and cerebrovascular accidents as well as the risk of death from myocardial infarction. Aspirin, 625 mg, is given to patients who have undergone coronary artery bypass grafting 6 hours after the procedure and daily for 1 year afterward. Table 14.4 presents specific dosage information for aspirin and other salicylates.
 TABLE 14.4
TABLE 14.4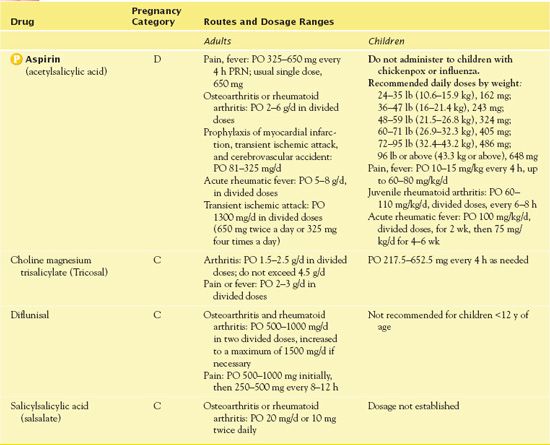
Use in Children
Aspirin is not recommended because of its association with Reye’s syndrome, a life-threatening illness characterized by encephalopathy, hepatic damage, and other serious problems. This syndrome usually occurs after a viral infection, such as influenza or chickenpox, during which aspirin was given for fever.
Use in Older Adults
Aspirin is safe in therapeutic doses for analgesic and antipyretic use. In addition, it is usually safe in the low doses prescribed for prevention of myocardial infarctions and cerebrovascular accidents.
Use in Patients With Renal Impairment
Aspirin is nephrotoxic in high doses, and protein binding of aspirin is reduced in patients with renal failure, which means that blood levels of the active drug are higher than they would be otherwise. Aspirin can also decrease the blood flow in the kidneys by inhibiting the synthesis of prostaglandins that dilate renal blood vessels. When renal blood flow is normal, these prostaglandins have limited activity. However, when renal blood flow is decreased, synthesis of these prostaglandins is increased, and they protect the kidneys from ischemia and hypoxia by antagonizing the vasoconstrictive effects of angiotensin II, norepinephrine, and other substances. Thus, in patients who depend on prostaglandins to maintain an adequate renal blood flow, the prostaglandin-blocking effects of aspirin result in constriction of renal arteries and arterioles, decreased renal blood flow, decreased glomerular filtration rate, and retention of salt and water.
Adverse Effects
GI adverse effects of aspirin include nausea, dyspepsia, heartburn, and epigastric discomfort. Decreased platelet aggregation results in GI blood loss and hemorrhage. In addition, petechiae and bruising may also occur. Aspirin toxicity occurs at levels above 300 mcg/mL. Acute toxicity results in respiratory alkalosis, hyperpnea, tachypnea, hemorrhage, confusion, pulmonary edema, seizures, tetany, metabolic acidosis, fever, coma, and cardiovascular collapse. Renal and respiratory failure occurs with doses of 20 to 25 g in adults and 4 g in children. Salicylism, toxicity due to salicylates that may be associated with chronic use, is characterized by dizziness, tinnitus, difficulty hearing, and mental confusion.
Contraindications
Aspirin is contraindicated in patients with a known sensitivity to aspirin; in those who are allergic to tartrazine, due to a cross-sensitivity; and in those with a known risk of bleeding. The U.S. Food and Drug Administration (FDA) has issued a BLACK BOX WARNING ♦ stating that children or teenagers should not take aspirin to treat chickenpox or flu-like symptoms because of the risk of Reye’s syndrome. This condition is a potentially fatal disease characterized by encephalopathy and fatty liver accumulations. It is associated with the use of aspirin and NSAIDs in children and adolescents after viral infections such as chickenpox or influenza.
Aspirin should be administered cautiously to patients with impaired renal function. Teratogenic effects of aspirin have been reported, and the drug should not be administered during pregnancy. Low-birth-weight infants, increased intracranial bleeding, and stillbirth have been reported in infants of mothers who took aspirin late in pregnancy.
Nursing Implications
Preventing Interactions
Medications and herbs interact with aspirin, increasing or decreasing its effects (Boxes 14.2 and 14.3).
BOX 14.2  Drug Interactions: Aspirin
Drug Interactions: Aspirin
Drugs That Increase the Effects of Aspirin
 Acidifying agents (e.g., vitamin C)
Acidifying agents (e.g., vitamin C)
Acidify urine and thereby decrease the urinary excretion rate of salicylates
 Anticoagulants, oral
Anticoagulants, oral
Increase the risk of bleeding; patients taking anticoagulants should not take aspirin.
 Codeine, hydrocodone, oxycodone
Codeine, hydrocodone, oxycodone
Have additive analgesic effects due to mechanism of action
 Corticosteroids
Corticosteroids
Have additive gastric irritation and possible ulcerogenic effects
Drugs That Decrease the Effects of Aspirin
 Alkalinizing agents (e.g., sodium bicarbonate)
Alkalinizing agents (e.g., sodium bicarbonate)
Increase the rate of renal excretion
 Ibuprofen
Ibuprofen
Competes with aspirin for COX-1 inhibition Negates the cardioprotective benefits of low-dose aspirin
 Misoprostol
Misoprostol
Prevents aspirin-induced gastric ulcers
BOX 14.3  Herb and Dietary Interactions: Aspirin
Herb and Dietary Interactions: Aspirin
Herbs and Foods That Increase the Effects of Aspirin
 Alcohol
Alcohol
 Gingko
Gingko
Administering the Medication
Aspirin should be taken with a full glass of water or other fluid and with food or just following food. Administering the medication with food decreases gastric irritation. Although the crushing of tablets or capsules results in faster absorption, this action destroys the long-acting feature and increases the risk of adverse effects and toxicity.
The dose of aspirin given depends mainly on the condition being treated. Low doses (325 mg initially and 80 mg daily) are used for the drug’s antiplatelet effects in preventing arterial thrombotic disorders such as myocardial infarction and stroke. Because aspirin is highly protein bound, lower-than-average doses are needed for patients with low serum albumin levels, because a larger proportion of each dose is free to exert pharmacologic activity. Larger doses are needed for anti-inflammatory effects (maximum daily dosage, 8000 mg) than for analgesic and antipyretic effects (325-650 mg every 4 hours). In general, patients taking low-dose aspirin to prevent myocardial infarction or stroke should continue to take the aspirin if their prescribers order a COX-2-inhibiting NSAID because the COX-2 inhibitors have little effect on platelet function.
Assessing for Therapeutic Effects
When administering aspirin, the nurse assesses for the therapeutic effects of the drug. If aspirin is given for pain, the nurse uses a pain scale to assess the intensity of the patient’s pain, which should decrease. If the drug is given for fever, the nurse records the patient’s temperature every 2 to 4 hours and should see a reduction in temperature. If the drug is given for inflammation, the nurse assesses for signs of inflammation, which should decrease. Patients receiving aspirin as a preventive agent for myocardial infarction or transient ischemic attack should be without chest pain or confusion.
Assessing for Adverse Effects
The nurse assesses the patient for bleeding tendencies, GI irritation, nausea, vomiting, and diarrhea, which all may result from aspirin use. The nurse assesses the skin for signs of decreased coagulation. The nurse performs a thorough pulmonary and integumentary assessment, looking for signs of hypersensitivity to aspirin, including dyspnea, bronchospasm, and rash.
Toxicity: Recognition and Management
Salicylate intoxication (salicylism) may occur with an acute overdose or with chronic use of therapeutic doses, especially the higher doses taken for anti-inflammatory effects. Chronic ingestion of large doses saturates a major metabolic pathway, thereby slowing drug elimination, prolonging the serum half-life, and causing drug accumulation. The therapeutic serum level of salicylate is 100 to 300 mcg/mL for the treatment of arthritis and rheumatic fever. Toxicity occurs at levels above 300 mcg/mL.
As previously stated, salicylism is characterized by dizziness, tinnitus, difficulty hearing, and mental confusion. Additional manifestations include nausea, vomiting, fever, fluid and electrolyte deficiencies, visual changes, drowsiness, hyperventilation, and other conditions. Severe central nervous system (CNS) dysfunction (e.g., delirium, stupor, coma, seizures) indicates life-threatening toxicity.
Treatment of Overdose
In mild salicylate toxicity, stopping the drug or reducing the dose is usually sufficient. In severe salicylate overdose, treatment is symptomatic and aimed at preventing further absorption from the GI tract, increasing urinary excretion, and correcting fluid, electrolyte, and acid-base imbalances. When the drug may still be in the GI tract, gastric lavage and activated charcoal help reduce absorption. Intravenous (IV) sodium bicarbonate produces an alkaline urine in which salicylates are more rapidly excreted, and hemodialysis effectively removes salicylates from the blood. IV fluids are indicated when high fever or dehydration is present. The specific content of IV fluids depends on the serum electrolyte and acid-base status.
Patient Teaching
Box 14.4 presents patient teaching guidelines for aspirin.
BOX 14.4  Patient Teaching Guidelines for Aspirin
Patient Teaching Guidelines for Aspirin
 Keep aspirin out of the reach of children.
Keep aspirin out of the reach of children.
 Use the drug as directed and do not overadminister the medication due to the risk of toxicity.
Use the drug as directed and do not overadminister the medication due to the risk of toxicity.
 Take the medication with food or after meals to prevent stomach upset.
Take the medication with food or after meals to prevent stomach upset.
 Do not crush or chew enteric-coated or sustained-release tablets.
Do not crush or chew enteric-coated or sustained-release tablets.
 Aspirin is as effective as more costly medication.
Aspirin is as effective as more costly medication.
 Watch for bleeding, ringing in the ears, or diminished hearing.
Watch for bleeding, ringing in the ears, or diminished hearing.
 Understand that fever is one way the body fights infection. Taking the medication for fever is not usually recommended unless the fever is high or is accompanied by other symptoms.
Understand that fever is one way the body fights infection. Taking the medication for fever is not usually recommended unless the fever is high or is accompanied by other symptoms.
 Avoid aspirin for approximately 2 weeks before and after major surgery or dental procedures to decrease the risk of excessive bleeding. If pregnant, do not take aspirin for approximately 2 weeks before the estimated delivery date.
Avoid aspirin for approximately 2 weeks before and after major surgery or dental procedures to decrease the risk of excessive bleeding. If pregnant, do not take aspirin for approximately 2 weeks before the estimated delivery date.
 Inform a health care provider if you have ever had an allergic reaction (e.g., asthma, difficulty breathing, hives), severe gastrointestinal symptoms (e.g., ulcer, bleeding), or rash or other skin disorder after taking aspirin.
Inform a health care provider if you have ever had an allergic reaction (e.g., asthma, difficulty breathing, hives), severe gastrointestinal symptoms (e.g., ulcer, bleeding), or rash or other skin disorder after taking aspirin.
 Avoid and minimize ingestion of alcohol due to gastric irritation and risk of bleeding.
Avoid and minimize ingestion of alcohol due to gastric irritation and risk of bleeding.



