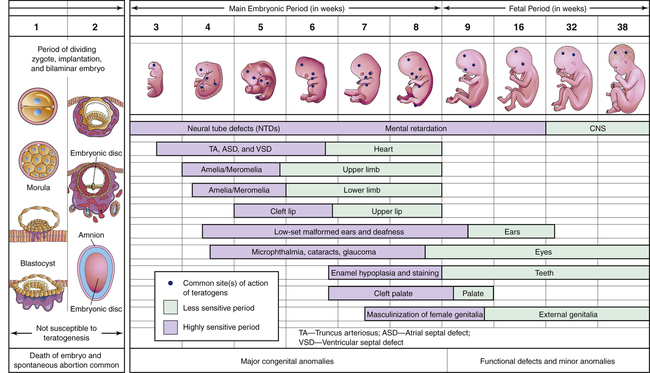
CHAPTER 9 Fetal sensitivity to teratogens changes during development, and hence the effect of a teratogen is highly dependent upon when the drug is given. As shown in Figure 9–1, development occurs in three major stages: the preimplantation/presomite period (conception through week 2), the embryonic period (weeks 3 through 8), and the fetal period (week 9 through term). During the preimplantation/presomite period, teratogens act in an “all-or-nothing” fashion. That is, if the dose is sufficiently high, the result is death of the conceptus. Conversely, if the dose is sublethal, the conceptus is likely to recover fully. For the following reasons, human teratogens are extremely difficult to identify: • The incidence of congenital anomalies is generally low. • Animal tests may not be applicable. • Prolonged exposure may be required. • Teratogenic effects may be delayed. • Behavioral effects are difficult to document. As a result, only a few drugs are considered proven teratogens. Drugs whose teratogenicity has been documented (or at least is highly suspected) are listed in Table 9–1. It is important to note, however, that lack of proof of teratogenicity does not mean that a drug is safe; it only means that the available data are insufficient to make a definitive judgment. Conversely, proof of teratogenicity does not mean that every exposure will result in a birth defect. In fact, with most teratogens, the risk of malformation following exposure is only about 10%. TABLE 9–1 Drugs That Should Be Avoided During Pregnancy Because of Proven or Strongly Suspected Teratogenicity*
Drug therapy during pregnancy and breast-feeding
Drug therapy during pregnancy: teratogenesis
Teratogenesis and stage of development
Identification of teratogens

Drug
Teratogenic Effect
Anticancer/Immunosuppressant Drugs
Cyclophosphamide
CNS malformation, secondary cancer
Methotrexate
CNS and limb malformations
Antiseizure Drugs
Carbamazepine
Neural tube defects
Valproic acid
Neural tube defects
Phenytoin
Growth retardation, CNS defects
Sex Hormones
Androgens (eg, danazol)
Masculinization of the female fetus
Diethylstilbestrol
Vaginal carcinoma in female offspring
Antimicrobials
Nitrofurantoin
Abnormally small or absent eyes, heart defects, cleft lip with cleft palate
Sulfonamides
Anencephaly, heart defects, transverse limb deficiency, diaphragmatic hernia
Tetracycline
Tooth and bone anomalies
Other Drugs
Alcohol
Fetal alcohol syndrome, stillbirth, spontaneous abortion, low birth weight, mental retardation
Angiotensin-converting enzyme inhibitors
Renal failure, renal tubular dysgenesis, skull hypoplasia (from exposure during the second and third trimesters)
Antithyroid drugs (propylthiouracil, methimazole)
Goiter and hypothyroidism
Nonsteroidal anti-inflammatory drugs
Premature closure of the ductus arteriosus
Lithium
Ebstein’s anomaly (cardiac defects)
Oral hypoglycemic drugs (eg, tolbutamide)
Neonatal hypoglycemia
Isotretinoin and other vitamin A derivatives (etretinate, megadoses of vitamin A)
Multiple defects (CNS, craniofacial, cardiovascular, others)
Thalidomide
Shortened limbs, internal organ defects
Warfarin
Skeletal and CNS defects ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Drug therapy during pregnancy and breast-feeding
Only gold members can continue reading. Log In or Register to continue
Get Clinical Tree app for offline access