Describe major characteristics and manifestations of Parkinson’s disease.
 Understand the pathophysiology of Parkinson’s disease.
Understand the pathophysiology of Parkinson’s disease.
 Describe the types of commonly used antiparkinson drugs.
Describe the types of commonly used antiparkinson drugs.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the dopamine receptor agonists.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the dopamine receptor agonists.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the catechol-O-methyltransferase (COMT) inhibitors.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the catechol-O-methyltransferase (COMT) inhibitors.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for a COMT inhibitor and decarboxylase inhibitor/dopamine precursor.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for a COMT inhibitor and decarboxylase inhibitor/dopamine precursor.
 Implement the nursing process in the care of patients undergoing drug therapy for Parkinson’s disease.
Implement the nursing process in the care of patients undergoing drug therapy for Parkinson’s disease.
 Describe the general characteristics of anticholinergic drugs.
Describe the general characteristics of anticholinergic drugs.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for belladonna alkaloids and derivatives.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for belladonna alkaloids and derivatives.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for centrally acting anticholinergic drugs.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for centrally acting anticholinergic drugs.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for anticholinergic medications used for gastrointestinal and urinary disorders.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for anticholinergic medications used for gastrointestinal and urinary disorders.
 Implement the nursing process in the administration of anticholinergic agents.
Implement the nursing process in the administration of anticholinergic agents.
Clinical Application Case Study
Lee Stokes is a 61-year-old man who visits his primary health care provider. He is experiencing pill-rolling movement of the right hand and fingers; slow, stooped movement; a shuffling gait with absence of arm movement; and excessive salivation. His physician diagnoses Mr. Stokes with Parkinson’s disease and starts him on levodopa/carbidopa (Sinemet) 25 mg carbidopa/100 mg levodopa four times a day and benztropine mesylate at bedtime.
KEY TERMS
Akinesia: rigid limbs
Anticholinergic drug: drug that inhibits the actions of acetylcholine in the brain
Antimuscarinic drug: drug that interacts with muscarinic cholinergic receptors in the brain, secretory glands, heart, and smooth muscle to produce an anticholinergic response
Basal ganglia: area in the midbrain that controls smooth voluntary movement
Bradykinesia: inability to move
Catechol-O-methyltransferase inhibitor: medication that inhibits the metabolism of levodopa in the periphery
Cycloplegia: Paralysis in the ciliary muscle of the eye
Dopamine receptor agonist: drug that corrects the neurotransmitter imbalance by increasing levels of dopamine
Extrapyramidal reactions: movement disorders such as tardive dyskinesia (inability to initiate movement), akathisia (inability to remain motionless), dystonia, and drug-induced parkinsonism that may occur with use of antiparkinsonism and antipsychotic drugs
Hypertensive crisis: severe increase in blood pressure that can lead to a stroke
Muscarinic receptors: located in the most internal organs, including the cardiovascular, respiratory, gastrointestinal (GI), and genitourinary systems. When activated by acetylcholine, the affected cells may be excited or inhibited in their functions
Mydriasis: pupil dilation
Nicotinic receptors: located in motor nerves and skeletal muscle; when activated by acetylcholine, the cell membrane depolarizes and produces muscle contraction
“Off time”: periods of the day when the medication is not working well, causing worsening of parkinsonian symptoms
Parkinson’s disease: chronic, progressive, degenerative disorder of the central nervous system characterized by resting tremor, bradykinesia, rigidity, and postural instability
Parkinsonism: often defined as a parkinsonian syndrome that is idiopathic (having no known cause), although some atypical cases have a genetic origin
Quaternary amines: anticholinergic drugs that carry a positive charge and are lipid insoluble; they do not readily cross the cell membranes, are poorly absorbed from the GI tract, and do not cross the blood–brain barrier
Substantia nigra: region of the midbrain with dopamine cells
Tertiary amines: anticholinergic drugs that are unchanged lipid-soluble molecules, able to cross cell membranes readily, and are well absorbed from the GI tract and conjunctiva, and they cross the blood–brain barrier
The first part of this chapter discusses Parkinson’s disease and the medications administered to decrease the symptoms of the disease. The second part discusses anticholinergic drugs administered to decrease secretions and prevent urinary urgency.
Overview of Parkinson’s Disease
Parkinson’s disease (also called parkinsonism) is a chronic, progressive, degenerative disorder of the central nervous system (CNS) characterized by resting tremor, bradykinesia, rigidity, and postural instability. Manifestations of Parkinson’s disease also may occur with other CNS diseases, brain tumors, and head injuries. Drugs that deplete dopamine stores or block dopamine receptors, including the older antipsychotic drugs (phenothiazines and haloperidol), reserpine, and metoclopramide, can produce movement disorders such as secondary parkinsonism (which also involves extrapyramidal reactions; see Chap. 55). Treatment can be pharmacologic, nonpharmacologic, and/or surgical.
The Parkinson’s Disease Foundation estimates that approximately 1 million people in the United States are living with Parkinson’s disease (2010a). This value includes about 60,000 people who are diagnosed each year with the disease, with 96% older than 50 years of age. Parkinson’s disease occurs slightly more often in men than in women and in Caucasian and Hispanic/Latino people than in African Americans.
Etiology
The cause of the nerve cell damage is unknown; age-related degeneration, genetics, and exposure to environmental toxins are possible etiologic factors. A total of nine genetic linkages and four genes have been associated with Parkinson’s disease, including mutations of alpha-synuclein and parkin genes. A high incidence of mutations in the parkin-2 gene has been associated with early-onset parkinsonism.
Pathophysiology
Idiopathic parkinsonism results from progressive destruction of or degenerative changes in dopamine-producing nerve cells in the substantia nigra in the basal ganglia, the area in the midbrain that controls smooth voluntary movement. The basal ganglia in the brain normally contain substantial amounts of the neurotransmitters dopamine and acetylcholine. The correct balance of dopamine and acetylcholine is important in regulating posture, muscle tone, and voluntary movement. People with Parkinson’s disease have an imbalance in these neurotransmitters, resulting in a decrease in inhibitory brain dopamine and a relative increase in excitatory acetylcholine.
Clinical Manifestations
The first symptom of Parkinson’s disease is often a resting tremor that begins in the fingers and thumb of one hand (“pill-rolling” movements), eventually spreading over one side of the body and progressing to the contralateral limbs. Other common symptoms include inability to move (bradykinesia), rigid limbs (akinesia), shuffling gait, stooped posture, mask-like facial expression, and a soft speaking voice. Less common manifestations may include depression, personality changes, loss of appetite, sleep disturbances, speech impairment, or sexual difficulty. Approximately 15% to 20% of people with Parkinson’s disease develop dementia. The severity of disease manifestations usually worsen over time. However, disease progression is often quite gradual, and patients may retain near-normal functional abilities for several years.
Drug Therapy
Drugs used in Parkinson’s disease include dopamine receptor agonists, which help correct the neurotransmitter imbalance by increasing levels of dopamine, and catechol-O-methyltransferase (COMT) inhibitors, which inhibit the metabolism of levodopa in the periphery. See Table 47.1. (The older belladonna alkaloids and the newer centrally acting anticholinergic agents inhibit the actions of acetylcholine in the brain. As previously stated, these medications are discussed later in this chapter.)
Clinical Application 47-1
 A nurse is providing patient teaching to Mr. Stokes and his family. The family inquires about the progression of Parkinson’s disease. What does the nurse tell the patient and the family with regard to disease progression?
A nurse is providing patient teaching to Mr. Stokes and his family. The family inquires about the progression of Parkinson’s disease. What does the nurse tell the patient and the family with regard to disease progression?
Dopamine Receptor Agonists
Levodopa (L-dopa), the original prototype dopamine receptor antagonist, was developed in the 1960s. It is routinely administered with the drug carbidopa; therefore, the combination medication is discussed as the prototype.  Levodopa/carbidopa (Sinemet, Sinemet CR, Parcopa) is well established as the most effective drug for the symptomatic treatment of idiopathic Parkinson’s disease. (Carbidopa is used only in conjunction with levodopa.) The combination is particularly effective for the management of akinetic symptoms.
Levodopa/carbidopa (Sinemet, Sinemet CR, Parcopa) is well established as the most effective drug for the symptomatic treatment of idiopathic Parkinson’s disease. (Carbidopa is used only in conjunction with levodopa.) The combination is particularly effective for the management of akinetic symptoms.
Pharmacokinetics
In peripheral tissues (e.g., gastrointestinal [GI] tract, liver), levodopa is metabolized extensively by the enzyme aromatic amino acid decarboxylase (AADC) and to a lesser extent by catechol-O-methyltransferase (COMT). Because most levodopa is metabolized in peripheral tissues, large doses are required to obtain therapeutic levels of dopamine in the brain. These large amounts increase adverse drug effects. To reduce levodopa dosage and decrease adverse effects, carbidopa, an AADC inhibitor, is given to decrease the peripheral metabolism of levodopa. The combination of levodopa and carbidopa greatly increases the amount of available levodopa, so that levodopa dosage can be reduced by approximately 70%. When carbidopa inhibits the decarboxylase pathway of levodopa metabolism, the COMT pathway becomes more important (see Catechol-O-Methyltransferase Inhibitors for a discussion of entacapone and tolcapone).
Levodopa is well absorbed from the small intestine after oral administration, reaches peak serum levels within 30 to 90 minutes, and has a short serum half-life (1-3 hours). Absorption is decreased by delayed gastric emptying, hyperacidity of gastric secretions, and competition with amino acids (from digestion of protein foods) for sites of absorption in the small intestine. Pyridoxine (vitamin B6) promotes the breakdown of levodopa, reducing its effectiveness. Levodopa is metabolized to 30 or more metabolites, some of which are pharmacologically active and probably contribute to drug toxicity; the metabolites are excreted primarily in the urine, usually within 24 hours.
Action
Dopaminergic drugs increase the amount of dopamine in the brain by various mechanisms (Fig. 47.1). If levodopa is administered alone, large doses must be taken to produce therapeutic effects. Carbidopa combined with levodopa prevents the decarboxylation of the levodopa, which makes levodopa more available for transportation to the brain. Levodopa is the metabolic precursor of dopamine, and after levodopa crosses the blood–brain barrier, it converts to dopamine in the brain. This is thought to be the mechanism whereby the drug relieves symptoms of Parkinson’s disease. Carbidopa does not cross the blood–brain barrier and does not affect levodopa metabolism.
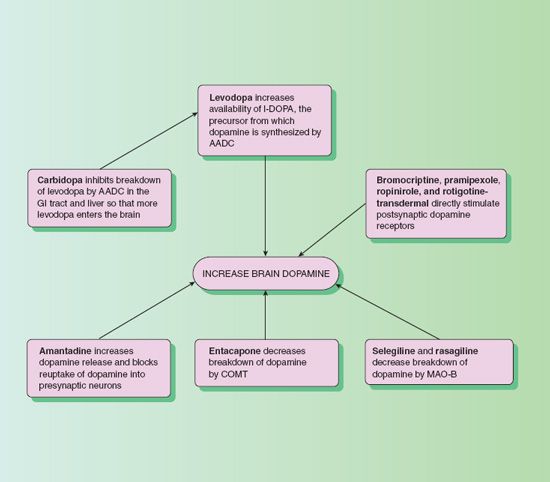
Figure 47.1 Mechanisms by which dopaminergic drugs increase dopamine in the brain. AADC, amino acid decarboxylase; COMT, catechol-O-methyltransferase; MAO-B, monoamine oxidase B.
Use
Levodopa/carbidopa is a treatment of idiopathic Parkinson’s disease, postencephalitic and arteriosclerotic parkinsonism, and parkinsonism related to carbon dioxide and manganese intoxication. Prescribers may also order levodopa to reduce the symptoms of restless leg syndrome (RLS). People with RLS, also known as Ekbom’s syndrome, experience paresthesias of the muscles, particularly in the calf and thighs, creating the urge to move. Movement relieves the paresthesia, which returns when the person is at rest or trying to sleep. The disorder may result in insomnia; mental distress; and, in some cases, suicide.
Levodopa and carbidopa are usually given together in a fixed-dose formulation called Sinemet. Table 47.2 gives doses of this combination and other dopamine receptor agonists used to treat Parkinson’s disease.
 TABLE 47.2
TABLE 47.2
Use in Children
Safety and effectiveness for use in children have not been established for most antiparkinson drugs. Parkinsonism is a degenerative disorder of adults, and antiparkinson drugs are most likely to be used in children for other purposes such as the stimulation of growth hormone in children with Down’s syndrome.
Use in Older Adults
It may be necessary to reduce dosages of levodopa/carbidopa because of an age-related decrease in peripheral AADC, the enzyme that carbidopa inhibits. The risk of hallucinations is increased in older patients who take dopamine agonist drugs.
Use in Patients With Renal Impairment
Caution is necessary with the use of levodopa/carbidopa in patients with renal failure. Dosage adjustments are required.
Use in Patients With Hepatic Impairment
With levodopa, cautious use in patients with hepatic impairment is warranted, and dosage reduction may be necessary. Reduced dosages are indicated with severe hepatic impairment. It is important to monitor liver transaminase enzymes frequently to assess for liver impairment. At the earliest sign of hepatotoxicity, drug withdrawal is essential, and there should be no reinstatement.
Use in Patients With Critical Illness
Caution is necessary with the use of levodopa in patients with severe neurological, cardiac, or hepatic injuries. Dosage adjustment to the lowest level required for therapeutic effects is essential.
Use in Patients Receiving Home Care
The home care nurse can help patients and caregivers understand that the purpose of drug therapy is to control symptoms and that noticeable improvement may not occur for several weeks. Also, the nurse can encourage patients to consult physical therapists, speech therapists, and dietitians to help maintain their ability to perform activities of daily living. In addition, teaching about preventing or managing adverse drug effects may be necessary. Caregivers may need to be informed that most activities (e.g., eating, dressing) take longer and require considerable effort by patients with parkinsonism.
Adverse Effects
Because of the adverse effects and recurrence of parkinsonism symptoms after a few years of levodopa therapy, levodopa is usually reserved for patients with significant symptoms and functional disabilities. The most common CNS adverse effects are headache and anxiety. Older patients may experience problems such as hallucinations, dementia, and drowsiness. The most severe adverse effect is depression with suicidal tendencies.
Cardiovascular adverse effects include ectopic beats, tachycardia, anginal pain, palpitations, hypotension, vasoconstriction, dyspnea, bradycardia, and a widened QRS. The medication can cause orthostatic hypotension. This effect is common during the first few weeks but usually subsides. Concurrent administration of nonselective monoamine oxidase (MAO) inhibitors (used in the treatment of depression) and levodopa can result in extreme elevations in blood pressure or hypertensive crisis. (MAO exists in two types, MAO-A and MAO-B, both of which are found in the CNS and peripheral tissues.)
In addition, some patients report anorexia, bruxism, and nausea and vomiting. Other less common adverse effects are piloerection, azotemia, and gangrene with prolonged use. Dermatologic effects such as hypersensitivity, anaphylaxis, and urticaria occur less frequently.
Contraindications
Contraindications to the use of levodopa/carbidopa include a known hypersensitivity to the drug. The drug can dilate pupils and raise intraocular pressure; thus, narrow-angle glaucoma is also a contraindication. Levodopa may activate malignant melanoma; people with suspicious skin lesions or a history of melanoma should not take it. To avoid the severe hypertension that may occur with concurrent use of some MAO inhibitors and levodopa, it is essential that MAO inhibitors be discontinued 14 days prior to beginning levodopa therapy. In addition, use of levodopa warrants caution in patients with severe cardiovascular, pulmonary, renal, hepatic, or endocrine disorders; depression; and peptic ulcer disease.
Nursing Implications
Preventing Interactions
The administration of levodopa/carbidopa with an MAO inhibitor can precipitate a hypertensive crisis. Postural hypotension occurs with the administration of tricyclic antidepressants and levodopa/carbidopa. Methyldopa combined with levodopa increases CNS effects. Dysrhythmic effects are increased when combined with halogenated general anesthetics. Several drugs interact with levodopa/carbidopa, increasing or decreasing its effects (Box 47.1). A high-protein meal increases the effects of levodopa/carbidopa, and kava decreases the effects of the drug.
BOX 47.1  Drug Interactions: Levodopa/Carbidopa
Drug Interactions: Levodopa/Carbidopa
Drugs That Increase the Effects of Levodopa/Carbidopa
 Monoamine oxidase inhibitors
Monoamine oxidase inhibitors
Increase the risk of hypertensive crisis
Drugs That Decrease the Effect of Levodopa/Carbidopa
 Anticholinergics
Anticholinergics
Increase anticholinergic effects by delaying gastric emptying
 Pyridoxine (vitamin B6)
Pyridoxine (vitamin B6)
Stimulates decarboxylase, the enzyme that converts levodopa to dopamine, causing metabolism in the peripheral tissues and decreasing medication distribution to the central nervous system
 Phenytoin, papaverine, tricyclic antidepressants, benzodiazepines
Phenytoin, papaverine, tricyclic antidepressants, benzodiazepines
Decrease drug efficacy
Administering the Medication
The nurse ensures that:
• Levodopa/carbidopa is administered with or just after food or following a meal to reduce nausea and vomiting.
• Sinemet CR is not crushed.
• Levodopa is not given with iron preparations or multivitamin–mineral preparations that contain iron.
• Levodopa/carbidopa is not administered with a high-protein diet. Adequate hydration is also necessary.
In addition, the nurse should ensure a temperature-controlled environment; this prevents hyperpyrexia.
QSEN Safety Alert 
When administering levodopa, carbidopa, and other medications for Parkinson’s disease, it is important that medications be given to the patient on time. Timing of medication administration is critical for optimal therapeutic effect.
Assessing for Therapeutic Effects
With levodopa and other dopaminergic agents, the nurse observes for improvement in mobility, balance, posture, gait, speech, handwriting, and self-care ability. Elimination of drooling and seborrhea may occur. Mood elevation may result. After 2 to 5 years, the medication may lose its overall effectiveness, and the dosage may need to be increased. The nurse needs to be aware of symptoms such as ataxic gait, tremors of the hands and fingers, drooling, and mask-like facial expressions.
Assessing for Adverse Effects
The nurse assesses for anorexia, nausea, and vomiting. These symptoms usually disappear after a few months of levodopa/carbidopa therapy. As previously stated, giving the drug with food minimizes these effects. The nurse also assesses the patient’s blood pressure in the sitting and standing positions to identify signs of orthostatic hypotension. This effect, too, commonly dissipates a few weeks after beginning therapy. Levodopa and its metabolites stimulate beta-adrenergic receptors in the heart. Patients with preexisting coronary artery disease may take propranolol (Inderal) to counteract cardiac dysrhythmia effects. It is necessary to assess the patient for dyskinesia. The involuntary movements of the tongue, mouth, and face are common adverse effects. Decreasing the dose of the medication decreases dyskinesia.
Patient Teaching
Box 47.2 identifies patient teaching guidelines for levodopa/carbidopa.
BOX 47.2  Patient Teaching Guidelines for Levodopa/Carbidopa
Patient Teaching Guidelines for Levodopa/Carbidopa
 Take the medication as prescribed.
Take the medication as prescribed.
 Do not crush the sustained-release preparation.
Do not crush the sustained-release preparation.
 Do not take multivitamin preparations containing pyridoxine.
Do not take multivitamin preparations containing pyridoxine.
 Understand that there are adverse effects of medication such as drowsiness, dizziness, and orthostatic hypotension.
Understand that there are adverse effects of medication such as drowsiness, dizziness, and orthostatic hypotension.
 Change positions slowly to prevent drop in blood pressure.
Change positions slowly to prevent drop in blood pressure.
 Avoid alcohol.
Avoid alcohol.
 Take the medication with food to prevent nausea and vomiting.
Take the medication with food to prevent nausea and vomiting.
 Do not take the medication with a high-protein meal.
Do not take the medication with a high-protein meal.
 Report fainting, light-headedness, irregular heart rate, uncontrolled facial movements, urinary retention, nausea, and vomiting to the prescriber.
Report fainting, light-headedness, irregular heart rate, uncontrolled facial movements, urinary retention, nausea, and vomiting to the prescriber.
 Notify the prescriber of any increase in symptoms such as static gait, altered mobility, and “pill rolling.”
Notify the prescriber of any increase in symptoms such as static gait, altered mobility, and “pill rolling.”
Other Drugs in the Class
Amantadine hydrochloride (Endantadine, Symmetrel) is an antiparkinson and antiviral agent (see Chap. 21). It increases the dopamine release in the nigrostriatal pathway of patients with Parkinson’s disease. It is absorbed in the GI tract with an onset of action of 36 to 48 hours, a peak of action of 1.5 to 8 hours, and a half-life of 10 to 25 hours. It crosses the placenta and enters the breast milk. It is excreted unchanged in the urine. The most common adverse effects of amantadine are dizziness, light-headedness, and insomnia. The nurse instructs the patient to report swelling of the fingers or ankles, difficulty walking, urinary retention, tremors, slurred speech, or thoughts of suicide to the health care provider. It is important not to discontinue this drug abruptly.
Apomorphine hydrochloride (Apokyn) is an antiparkinson agent administered for “off time,” or “off” episodes, of Parkinson’s disease—to assist in diminishing the symptoms of hypomobility. “Off time” is the period when the medication is not adequately controlling the patient’s symptoms. Patients who suffer from “off time” episodes have advanced Parkinson’s disease. Administration is subcutaneous. Doses are incremental, generally ranging from 20 to 40 mg. The most common dosage is 30 mg, or 0.3 mL, and the maximum dosage is 60 mg. The patient’s blood pressure must be monitored for hypertensive crisis during the administration. When apomorphine is administered to patients with a known cardiac history, periodic electrocardiogram results should be monitored as well as serum electrolytes.
Bromocriptine mesylate (Parlodel, Cycloset) is an ergot derivative that directly stimulates dopamine receptors in the brain. It is used in the treatment of idiopathic Parkinson’s disease, with levodopa/carbidopa, to prolong effectiveness and to allow reduced dosage of levodopa. Administration to patients with a history of myocardial infarction with residual dysrhythmia requires caution.
Cabergoline, a synthetic ergot, is a long-acting dopamine agonist approved by the U.S. Food and Drug Administration (FDA) for use in hyperprolactinemia. This medication has an unlabeled use: to improve motor symptoms of parkinsonism. However, the higher dosage required to treat parkinsonism is associated with the development of serious heart valve damage due to fibrosis.
Pramipexole (Mirapex) and ropinirole (Requip) stimulate dopamine receptors in the brain. The FDA has approved their use in both early and late stages of Parkinson’s disease. In early stages, one of these drugs can be used alone to improve motor performance, improve ability to participate in usual activities of daily living, and delay levodopa therapy. In advanced stages, one of these drugs can be used with levodopa and perhaps other antiparkinson drugs to provide more consistent relief of symptoms between doses of levodopa and allow reduced dosage of levodopa. These drugs, which are not ergot derivatives, may not cause some adverse effects associated with bromocriptine (e.g., pulmonary and peritoneal fibrosis, constriction of coronary arteries).
Pramipexole is rapidly absorbed with oral administration. Peak serum levels are reached in 1 to 3 hours after a dose and steady-state concentrations in about 2 days. The drug is less than 20% bound to plasma proteins and has an elimination half-life of 8 to 12 hours. Most of the drug is excreted unchanged in the urine; only 10% is metabolized. As a result, renal failure may cause higher-than-usual plasma levels and possible toxicity. However, hepatic disease is unlikely to alter drug effects.
Ropinirole is also well absorbed with oral administration. It reaches peak serum levels in 1 to 2 hours and steady-state concentrations within 2 days. It is 40% bound to plasma proteins and has an elimination half-life of 6 hours. It is metabolized by the cytochrome P450 enzymes in the liver to inactive metabolites, which are excreted through the kidneys. Less than 10% of ropinirole is excreted unchanged in the urine. Thus, hepatic failure may decrease metabolism, allow drug accumulation, and increase adverse effects. Renal failure does not appear to alter drug effects.
Rasagiline (Azilect) is an irreversible MAO inhibitor. It is indicated for initial treatment for idiopathic parkinsonism and as an adjunct therapy with levodopa to reduce “off time” when movements are poorly controlled. Because it has not been determined to be selective for MAO-B in humans, care must be taken to avoid tyramine-containing foods as well as sympathomimetic medications to prevent hypertensive crisis. In addition, rasagiline has the potential to increase serotonin neurotransmission. When given with other drugs that enhance stimulation of serotonergic receptors (e.g., antidepressants, St. John’s wort, dextromethorphan, and meperidine), serotonin syndrome, a potential fatal CNS toxicity reaction characterized by hyperpyrexia and death, can occur. Rasagiline should be discontinued at least 14 days before beginning treatment with most antidepressants or other MAO inhibitors. Fluoxetine should be discontinued at least 5 weeks before initiating rasagiline, due to its long half-life. Rasagiline is well absorbed orally, metabolized in the liver, and excreted primarily by the kidney. It is contraindicated with foods containing tyramine or sympathomimetic amine-containing medications (e.g., nonprescription cold preparations and anesthetics) because of the risk of hypertensive crisis and with antidepressants (e.g., tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, mirtazapine), meperidine, and dextromethorphan because of the potential for inducing serotonin syndrome.
Selegiline (Eldepryl) inhibits metabolism of dopamine by MAO, which exists in two types (as previously stated). These types are differentiated by their relative specificities for individual catecholamines. MAO-A acts more specifically on tyramine, norepinephrine, epinephrine, and serotonin. This enzyme is the main subtype in GI mucosa and in the liver and is responsible for metabolizing dietary tyramine. If MAO-A is inhibited in the intestine, tyramine in various foods is absorbed systemically rather than deactivated. As a result, there is excessive stimulation of the sympathetic nervous system, and severe hypertension and stroke can occur. This is sometimes called the “cheese reaction” because aged cheeses are high in tyramine. This life-threatening reaction can also occur with some medications (e.g., sympathomimetics) that are normally metabolized by MAO. MAO-B metabolizes dopamine; in the brain, most MAO activity is due to type B. At oral dosages of 10 mg/d or less, selegiline inhibits MAO-B selectively and is unlikely to cause severe hypertension and stroke. However, at dosages greater than 10 mg/d, selectivity is lost and metabolism of both MAO-A and MAO-B is inhibited. Dosages greater than 10 mg/d should be avoided in patients with Parkinson’s disease. Selegiline inhibition of MAO-B is irreversible, and drug effects persist until more MAO is synthesized in the brain, which may take several months.
In early Parkinson’s disease, selegiline may be effective as monotherapy (level A). In advanced disease, prescribers order the drug to enhance the effects of levodopa. Its addition aids symptom control and allows the dosage of levodopa/carbidopa to be reduced. Once proposed to have neuroprotective properties, authorities now believe that there is insufficient evidence to recommend the use of selegiline to confer neuroprotection in patients with Parkinson’s disease (level U).
NCLEX Success
1. The daughter of a 75-year-old woman states to the parish nurse that she has noticed her mother rolling her fingers together on her right hand. The nurse observes the patient and determines she is “pill rolling,” which is characteristic of Parkinson’s disease. Which of the following factors contributes to the development of central nervous system symptom of “pill rolling”?
A. decrease firing of the sinoatrial node
B. conversion of angiotensin I to angiotensin II
C. increase in excitatory acetylcholine
D. influx of potassium through the cell membrane
2. A 65-year-old woman has been taking levodopa for several weeks for symptoms of Parkinson’s disease. Which of the following symptoms indicates that she is not receiving an adequate dose for the treatment of her symptoms?
A. edema of the feet and ankles
B. widened QRS complex
C. static gait
D. increased intraocular pressure
3. The 56-year-old man is taking levodopa/carbidopa for Parkinson’s disease. During the therapy, he becomes light-headed and dizzy. Which of the following is a potentially serious adverse effect of the drug treatment?
A. orthostatic hypotension
B. diminished fluid volume
C. hematuria
D. jaundice
4. A 52-year-old man is taking selegiline for the treatment of Parkinson’s disease. He consumes port wine cheese and crackers at a party. Which of the following symptoms does he develop?
A. ataxic gait
B. melena
C. cardiac dysrhythmia
D. hypertension
5. A 48-year-old man with severe akinesia develops severe symptoms of parkinsonism following the administration of his antiparkinson medications. This condition occurs only one to two times per month. Which of the following medications is the man’s prescriber most likely to order?
A. bromocriptine mesylate (Parlodel)
B. apomorphine hydrochloride (Apokyn)
C. cabergoline (Dostinex)
D. pramipexole dihydrochloride (Mirapex, Mirapex ER)
Clinical Application 47-2
 Mr. Stokes has been having “off time” symptom development. His neurologist orders rasagiline (Azilect). He develops an upper respiratory viral infection with a cough. He begins to take dextromethorphan hydrobromide (Robitussin) every 4 hours. What is Mr. Stokes at risk for developing?
Mr. Stokes has been having “off time” symptom development. His neurologist orders rasagiline (Azilect). He develops an upper respiratory viral infection with a cough. He begins to take dextromethorphan hydrobromide (Robitussin) every 4 hours. What is Mr. Stokes at risk for developing?
 What symptom does the nurse assess Mr. Stokes for when combining rasagiline and dextromethorphan hydrobromide?
What symptom does the nurse assess Mr. Stokes for when combining rasagiline and dextromethorphan hydrobromide?
Catechol-O-Methyl transferase Inhibitors
 Tolcapone (Tasmar) is the prototype COMT inhibitor. COMT plays a role in brain metabolism of dopamine and metabolizes approximately 10% of peripheral levodopa. By inhibiting COMT, tolcapone increases levels of dopamine in the brain and relieves symptoms more effectively and consistently.
Tolcapone (Tasmar) is the prototype COMT inhibitor. COMT plays a role in brain metabolism of dopamine and metabolizes approximately 10% of peripheral levodopa. By inhibiting COMT, tolcapone increases levels of dopamine in the brain and relieves symptoms more effectively and consistently.
Pharmacokinetics
Tolcapone is absorbed rapidly and is highly protein bound. It is metabolized in the liver and possesses a 2 to 3 hour half-life. It crosses the placenta and enters the breast milk. It is excreted in the feces and urine.
Action
The main mechanism of action of tolcapone seems to be inhibiting the metabolism of levodopa in the bloodstream, thus increasing the plasma concentration and duration of action of the drug. It may also inhibit COMT in the brain and prolong the activity of dopamine at the synapse.
Use
Tolcapone is useful for the treatment of signs and symptoms of idiopathic Parkinson’s disease. Administration is only in conjunction with levodopa/carbidopa, and a reduction in levodopa dosage is required. If a patient does not show a clinical benefit within 3 weeks of starting treatment, discontinuation of tolcapone is necessary.
Table 47.3 presents dosage information for tolcapone and other drugs in its class.
 TABLE 47.3
TABLE 47.3
Use in Older Adults
When administering tolcapone to geriatric patients, it is important to reduce the dosage and adjust it slowly to prevent adverse effects.
Use in Patients With Renal Impairment
Caution is warranted in administration of tolcapone to patients with renal impairment because it is excreted in the urine.
Use in Patients With Hepatic Impairment
If liver values are greater than two times the upper limit of normal, discontinuation of tolcapone is necessary. Patients with moderate to severe hepatic impairment should not take tolcapone at doses exceeding 100 mg three times per day. The FDA has issued a BLACK BOX WARNING ♦ stating that patients who take tolcapone risk potentially fatal acute fulminant liver failure. It is important to monitor liver function tests before therapy begins and every 2 weeks thereafter.
Adverse Effects
Tolcapone produces adverse effects in several major body systems, including the CNS, cardiovascular system, dermatological system, GI system, and respiratory system. The most severe adverse effect is fulminant liver failure, which may be fatal. CNS adverse effects include disorientation, confusion, hallucinations, and psychosis. Dizziness and orthostatic hypotension may also occur.
Contraindications
Contraindications to tolcapone include a hypersensitivity to the drug. Other contraindications are liver disease, nontraumatic rhabdomyolysis, hyperpyrexia, and confusion. Caution is warranted with hypertension, hypotension, and renal impairment.
Nursing Implications
Preventing Interactions
It is essential that tolcapone and other COMT medications not be administered with MAO inhibitors due to the risk of hypertensive crisis.
Administering the Medication
It is necessary to administer tolcapone in conjunction with levodopa/carbidopa and to monitor the patient’s response to the medication. The addition of the drug may require a decrease in the levodopa dosage. Abrupt withdrawal of tolcapone can lead to serious complications. Tapering over 2 weeks is necessary to prevent adverse effects.
Assessing for Therapeutic Effects
The decrease or absence of symptoms of Parkinson’s disease such as improved gait and mobility, diminished tremors, and rigidity is indicative of tolcapone’s therapeutic effectiveness.
Assessing for Adverse Effects
The nurse assesses for disorientation and confusion, lightheadedness, and orthostatic hypotension. It is necessary to take blood pressure lying down, sitting, and standing up. Frequent monitoring of liver enzymes is essential.
Patient Teaching
Box 47.3 presents patient teaching guidelines for tolcapone.
BOX 47.3  Patient Teaching Guidelines for Tolcapone
Patient Teaching Guidelines for Tolcapone



