Discuss male reproductive problems in terms of etiology, pathophysiology, and clinical manifestations.
 Describe the androgens in terms of prototype, mechanism of action, indications for use, major adverse effects, and nursing implications.
Describe the androgens in terms of prototype, mechanism of action, indications for use, major adverse effects, and nursing implications.
 Identify potential consequences of abusing androgens and anabolic steroids.
Identify potential consequences of abusing androgens and anabolic steroids.
 Discuss the phosphodiesterase type 5 inhibitors in terms of prototype, mechanism of action, indications for use, major adverse effects, and nursing implications.
Discuss the phosphodiesterase type 5 inhibitors in terms of prototype, mechanism of action, indications for use, major adverse effects, and nursing implications.
 Describe the 5-alpha reductase inhibitors in terms of prototype, mechanism of action, indications for use, major adverse effects, and nursing implications.
Describe the 5-alpha reductase inhibitors in terms of prototype, mechanism of action, indications for use, major adverse effects, and nursing implications.
 Describe the alpha-adrenergic blockers in terms of prototype, mechanism of action, indications for use, major adverse effects, and nursing implications.
Describe the alpha-adrenergic blockers in terms of prototype, mechanism of action, indications for use, major adverse effects, and nursing implications.
 Implement the nursing process for men who have reproductive disorders.
Implement the nursing process for men who have reproductive disorders.
Clinical Application Case Study
Phillip johnson, a 52-year-old veterinarian, is seen in the clinic for hypertension control. He has a one-pack per day history of cigarette smoking and has begun walking to reduce his weight. For 5 years, he has been taking hydrochlorothiazide and atenolol to lower his blood pressure, which is well controlled. He has no other chronic health problems.
However, Mr. Johnson reports significant erectile dysfunction that has progressed over the past 9 months. This has significantly distressed him and his wife and has caused considerable marital discord.
KEY TERMS
Anabolic steroids: synthetic drugs with increased anabolic activity and decreased androgenic activity compared with testosterone
Androgens: male sex hormones, primarily testosterone, secreted by the testes in men, the ovaries in women, and the adrenal cortex in both men and women
Benign prostatic hypertrophy: benign enlargement of the prostate gland; also known as benign prostatic hyperplasia
Corpora cavernosa: major erectile tissue of the penis
Erectile dysfunction: difficulty in initiating or maintaining penile erection that is satisfactory for sexual relations
Erectogenic: capable of causing an erection
Ergogenic: increase in muscular work capacity caused by drugs that enhance athletic performance
Leydig cells: cells in the testes that control androgen secretion
Testosterone: male sex hormone; secreted by the Leydig cells in the testes
Introduction
This chapter explores the drugs used to manage disorders and conditions that affect men’s reproductive health. To understand the drugs discussed in this chapter, it is necessary to understand the regulation of male reproductive function as well as recognize the main disorders of male reproduction.
Overview of Reproductive Health Problems in Men
Androgens are male sex hormones secreted by the testes in men, the ovaries in women, and the adrenal cortex in both men and women. Like the female sex hormones, the naturally occurring male sex hormones are steroids synthesized from cholesterol, which is why they are called anabolic steroids. The sex organs and adrenal glands can produce cholesterol or remove it from the blood. Cholesterol then undergoes a series of conversions to progesterone, androgenic prehormones, and testosterone. The androgens produced by the ovaries have little androgenic activity and are used mainly as precursor substances for the production of naturally occurring estrogens. The adrenal glands produce several androgens, including androstenedione and dehydroepiandrosterone (DHEA). Androstenedione and DHEA are weak androgens with little masculinizing effect that are mainly converted to estrogens.
Testosterone is normally the only important androgen. This male sex hormone has several effects on body tissues (Box 45.1). Certain cells in the testes, called Leydig cells, secrete testosterone in response to stimulation by luteinizing hormone (LH) from the anterior pituitary gland. The increased production of testosterone results in the changes associated with puberty in the adolescent male. The main functions of testosterone concern the development of male sexual characteristics, reproduction, and metabolism. Testosterone is necessary for normal sperm development.
BOX 45.1 Effects of Testosterone on Body Tissues
Fetal Development
Large amounts of chorionic gonadotropin are produced by the placenta during pregnancy. Chorionic gonadotropin is similar to luteinizing hormone (LH) from the anterior pituitary gland. It promotes development of the interstitial or Leydig cells in fetal testes, which then secrete testosterone. Testosterone production begins in the second month of fetal life. When present, testosterone promotes development of male sexual characteristics (e.g., penis, scrotum, prostate gland, seminal vesicles, and seminiferous tubules), and suppresses development of female sexual characteristics. In the absence of testosterone, the fetus develops female sexual characteristics.
Testosterone also provides the stimulus for the descent of the testes into the scrotum. This normally occurs after the seventh month of pregnancy, when the fetal testes are secreting relatively large amounts of testosterone. If the testes do not descend before birth, administration of testosterone or gonadotropic hormone, which stimulates testosterone secretion, produces descent in most cases.
Adult Development
Little testosterone is secreted in boys until 11 to 13 years of age. At the onset of puberty, testosterone secretion increases rapidly and remains at a relatively high level until about 50 years of age, after which it gradually declines.
The testosterone secreted at puberty acts as a growth hormone to produce enlargement of the penis, testes, and scrotum until approximately 20 years of age. The prostate gland, seminal vesicles, seminiferous tubules, and vas deferens also increase in size and functional ability. Under the combined influence of testosterone and follicle-stimulating hormone (FSH) from the anterior pituitary gland, sperm production is initiated and maintained throughout the man’s reproductive life. It affects various parts of the body.
 Skin. Testosterone increases skin thickness and activity of the sebaceous glands. Acne in the male adolescent is attributed to the increased production of testosterone.
Skin. Testosterone increases skin thickness and activity of the sebaceous glands. Acne in the male adolescent is attributed to the increased production of testosterone.
 Voice. The larynx enlarges and deepens the voice of the adult man.
Voice. The larynx enlarges and deepens the voice of the adult man.
 Hair. Testosterone produces the distribution of hair growth on the face, limbs, and trunk typical of the adult man. In men with a genetic trait toward baldness, large amounts of testosterone cause alopecia (baldness) of the scalp.
Hair. Testosterone produces the distribution of hair growth on the face, limbs, and trunk typical of the adult man. In men with a genetic trait toward baldness, large amounts of testosterone cause alopecia (baldness) of the scalp.
 Skeletal muscles. Testosterone is largely responsible for the larger, more powerful muscles of men. This characteristic is caused by the effects of testosterone on protein metabolism. Testosterone helps the body retain nitrogen, form new amino acids, and build new muscle protein. At the same time, it slows the loss of nitrogen and amino acids formed by the constant breakdown of body tissues. Overall, testosterone increases protein anabolism (buildup) and decreases protein catabolism (breakdown).
Skeletal muscles. Testosterone is largely responsible for the larger, more powerful muscles of men. This characteristic is caused by the effects of testosterone on protein metabolism. Testosterone helps the body retain nitrogen, form new amino acids, and build new muscle protein. At the same time, it slows the loss of nitrogen and amino acids formed by the constant breakdown of body tissues. Overall, testosterone increases protein anabolism (buildup) and decreases protein catabolism (breakdown).
 Bone. Testosterone makes bones thicker and longer. After puberty, more protein and calcium are deposited and retained in bone matrix. This causes a rapid rate of bone growth. The height of a male adolescent increases rapidly for a time, then stops as epiphyseal closure occurs. This happens when the cartilage at the end of the long bones in the arms and legs becomes bone. Further lengthening of the bones is then prevented.
Bone. Testosterone makes bones thicker and longer. After puberty, more protein and calcium are deposited and retained in bone matrix. This causes a rapid rate of bone growth. The height of a male adolescent increases rapidly for a time, then stops as epiphyseal closure occurs. This happens when the cartilage at the end of the long bones in the arms and legs becomes bone. Further lengthening of the bones is then prevented.
Anterior Pituitary Function
 High blood levels of testosterone decrease secretion of FSH and LH from the anterior pituitary gland. This, in turn, decreases testosterone production.
High blood levels of testosterone decrease secretion of FSH and LH from the anterior pituitary gland. This, in turn, decreases testosterone production.
About 97% of the testosterone secreted by the testes binds to plasma albumin or to sex hormone–binding globulin and circulates in the blood for 30 minutes to several hours. The bound testosterone is either transferred to the tissues or broken down into inactive products that are excreted. Much of the testosterone that transfers to tissues undergoes intracellular conversion to dihydrotestosterone (DHT), which combines with receptor proteins in the cytoplasm. A series of actions stimulates production of proteins. Almost all effects of testosterone result from the increased formation of proteins throughout the body, especially in the cells of target organs and tissues responsible for development of male sexual characteristics.
The portion of testosterone that does not become attached to tissues is converted into androsterone and DHEA by the liver. These are conjugated with glucuronic or sulfuric acid and excreted in the bile or urine.
Etiology
Androgen Deficiency
Lack of sufficient testosterone can result in congenital or acquired hypogonadism in the male. Causes include genetic diseases, head or testicular trauma, alkylating agents, tumors, and radiation injury.
• Primary hypogonadism results from a testicular disorder. Common diseases that can cause primary hypogonadism are mumps, testicular inflammation, and trauma.
• Secondary hypogonadism results from a problem in the hypothalamus or the pituitary gland, areas of the brain that signal the testicles to produce testosterone. A lack of production of gonadotropin-releasing hormone from the hypothalamus or a deficiency of follicle-stimulating hormone (FSH) and LH from the anterior pituitary may result from thyroid disorders, Cushing’s syndrome, or estrogen-secreting tumors. Chronic diseases (e.g., metabolic syndrome, diabetes) can lead to secondary hypogonadism.
Erectile Dysfunction
Erectile dysfunction (ED) is defined as difficulty initiating or maintaining penile erection that is satisfactory for sexual relations. The condition affects more than 50% of men between 40 and 70 years of age. Incidence increases with age. Causes may include drugs (antidepressants, antihypertensive agents, histamine receptor antagonists), lifestyle factors (alcohol, tobacco, or cocaine use), diseases (diabetes, thyroid conditions, prostate cancer, cardiovascular conditions, obesity), and spinal cord injuries. Psychological factors may also play a role. Low testosterone levels rarely lead to ED but may reduce a man’s sex drive. ED and cardiovascular disease share many risk factors; the pathophysiology of both conditions is mediated through endothelial dysfunction. Cardiovascular disease enhances the risk of developing ED; conversely, ED is thought to be a powerful predictor of coronary artery disease, especially in men older than 60 years of age.
Benign Prostatic Hypertrophy
Benign prostatic hypertrophy (BPH), also known as benign prostatic hyperplasia, is benign enlargement of the prostate gland. More than 50% of men in their 60s and as many as 90% in their 70s and 80s have some symptoms of BPH. Part of the male reproductive system, the prostate is a walnut-sized gland composed of two lobes enclosed by an outer layer of tissue. This gland produces part of the seminal fluid that nourishes and transports sperm. The prostate is located in front of the rectum and below the bladder, where urine is stored, and it surrounds the urethra, the canal through which urine passes out of the body.
It is thought that BPH is a normal element of the male aging process. Causes include changes in hormone balance and cell growth. Testosterone undergoes reduction to form the more potent androgen DHT, which has greater affinity for androgen receptors than testosterone. During the formation and growth of an embryo, DHT plays a critical role in the formation of the male external genitalia, whereas in the adult, DHT acts as the primary androgen in the prostate and in hair follicles.
Pathophysiology
Androgen Deficiency
• Hypogonadism results from dysfunction of the hypothalamus, the anterior pituitary, or the gonads. Chapter 41 describes the role of the hypothalamic–pituitary–adrenal axis. Primary hypogonadism involves pathology of either Leydig cells or the seminiferous tubules, or both. Injury to Leydig cells results in decreased testosterone production, whereas seminiferous tubule dysfunction results in decreased or absent spermatogenesis. The majority of the conditions causing testicular injury predominantly involve tubules. Gonadotropins are elevated due to loss of negative feedback.
• Secondary hypogonadism involves decreased or incorrectly normal gonadotropin levels, leading to decreased testicular stimulation, spermatogenesis, and androgen production and if prolonged, testicular atrophy.
Erectile Dysfunction
The process of achieving penile erection involves the integration of psychological, neurological, and vascular processes, which combine to initiate a physiologic response within the penile vasculature. Stimulation of penile shaft results in the secretion of nitric oxide, which causes the relaxation of the smooth muscles of the corpora cavernosa (the main erectile tissue of penis). This results in increased blood flow into the sinusoids of the corpora cavernosa with subsequent filling, which obstructs venous outflow from the penis by compression of the veins against the tunica albuginea, resulting in penile erection. Additionally, adequate levels of testosterone and an intact pituitary gland are required.
Benign Prostatic Hypertrophy
The prostate contains stromal and epithelial tissue. Stromal tissue is smooth muscle tissue that contracts around the urethra when activated and is regulated by alpha1-adrenergic receptors. Epithelial tissue is the glandular portion of the prostate and is under the control of androgens, primarily testosterone. The enzyme 5-alpha reductase converts testosterone to DHT, its active metabolite, in epithelial tissue.
The prostate undergoes two main growth periods, first in puberty, when the prostate doubles in size, and second in early adulthood, at about 25 years of age, when the gland begins another growth phase that often results years later in BPH. A surrounding layer of tissue limits overgrowth of the epithelial or glandular tissue of the prostate, causing the gland to put pressure against the urethra. In addition, the bladder wall becomes thicker and more easily irritated; it contracts even when it contains small amounts of urine, leading to more frequent urination. At some point, the bladder weakens and loses the capacity to empty, and urine is retained in the bladder. The narrowing of the urethra and partial emptying of the bladder cause many of the problems associated with BPH.
Clinical Manifestations
Androgen Deficiency
Signs and symptoms of androgen deficiency in adult males include ED, infertility, decreased beard and body hair growth, decreased muscle mass, breast tissue (gynecomastia), and loss of bone mass (osteoporosis). Hypogonadism can also cause mental and emotional changes. As testosterone decreases, some men may experience symptoms similar to those of menopause in women, including fatigue, decreased sex drive, difficulty concentrating, and hot flashes.
To confirm a diagnosis of hypogonadism, it is necessary to perform laboratory tests. Serum testosterone is decreased. To determine the cause, it is also necessary to measure hormone levels in the serum: FSH, LH, prolactin, thyroid hormone, and estradiol. If normal or elevated FSH and LH serum levels are present with a low testosterone level and the testes are non-responsive to hormonal stimulation, a primary hypogonadism is present. If FSH and LH are low along with the testosterone level, a secondary hypogonadism is present.
Erectile Dysfunction
The main clinical manifestation of ED is the consistent inability to attain or maintain an erection satisfactory for sexual activity. An associated symptom may be reduced sexual desire.
Benign Prostatic Hypertrophy
Clinical manifestations of BPH result from the obstruction of the urethra from the enlargement of the prostate gland, causing reduction in outflow of urine. Rarely do symptoms occur in men younger than 40 years of age. Men may experience urinary frequency, hesitancy, urgency, dribbling, and decrease force of the urinary stream. Nocturia, postvoid leakage, urinary stones, or infection can also occur.
On physical examination, a midline mass above the symphysis pubis, which likely represents an incompletely emptied bladder, may be visible. On digital rectal examination, rubbery enlargement of the prostate is present. Excretory urography may indicate emptying and filling defects in the bladder, urinary tract obstruction, calculi or tumors, and hydronephrosis.
Drug Therapy
Table 45.1 outlines the drugs used to maintain male reproductive health, which include the androgens and anabolic steroids, the phosphodiesterase type 5 (PDE5) inhibitors and prostaglandins, and the 5-alpha reductase inhibitors and the alpha1-adrenergic inhibitors.
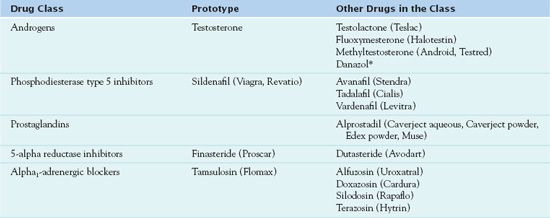
* Used in women
Androgens and Anabolic Steroids
When clinicians use male sex hormones or androgens from exogenous sources for therapeutic purposes, the effects are the same as those of naturally occurring hormones. These effects include inhibition of endogenous sex hormones and sperm formation through negative feedback of pituitary LH and FSH. Anabolic steroids are synthetic drugs with increased anabolic activity and decreased androgenic activity compared with endogenous testosterone. They were developed during attempts to modify testosterone so that its tissue-building and growth-stimulating effects could be retained while the drug’s masculinizing effects could be eliminated or reduced.
Prescribers may order male sex hormones for women to antagonize or reduce the effects of female sex hormones. Thus, administration of androgenic or anabolic steroids to women causes suppression of menstruation and atrophy of the endometrial lining of the uterus.
Several dosage forms of androgens are available. They differ mainly in route of administration and pharmacokinetics.  Testosterone is the prototype.
Testosterone is the prototype.
Pharmacokinetics
Like endogenous testosterone, the drug molecules are highly bound (98%) to plasma proteins and serum half-life varies (e.g., 8 days for IM testosterone cypionate, 9 hours for oral fluoxymesterone). Testosterone is extensively metabolized in its first pass through the liver, so that nearly half of a dose is lost before it reaches the systemic circulation. About 90% of a dose is excreted in urine as conjugates of testosterone and its metabolites; the remainder is excreted in feces.
Action
Like other steroid drugs, testosterone penetrates the cell membrane and binds to receptor proteins in the cell cytoplasm. The steroid–receptor complex is then transported to the nucleus, where it activates ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) production and stimulates cellular synthesis of protein.
Use
With testosterone, the most clear-cut indication for use is to treat androgen deficiency states (e.g., hypogonadism, cryptorchidism, impotence, oligospermia) in boys and men. In postpubertal men who become androgen deficient, the hormone reestablishes and maintains masculine characteristics and functions. Table 45.2 gives route and dosage information for testosterone and other androgens and anabolic steroids.
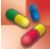 TABLE 45.2
TABLE 45.2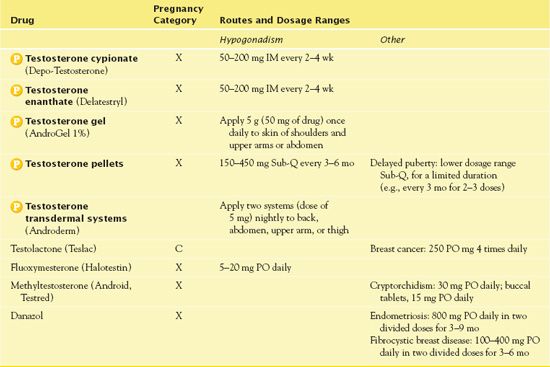
Use in Children
The main indication for use of testosterone in children is for boys with established sex hormone deficiencies; the hormone stimulates the development of masculine characteristics.
QSEN Safety Alert 
Because testosterone can cause epiphyseal closure, it is essential to examine a child’s hands and wrists using radiography every 6 months to detect bone maturation and to check that there is no loss of height if receiving testosterone therapy.
Stimulation of skeletal growth should continue for approximately 6 months after testosterone therapy stops. If premature puberty occurs (e.g., precocious sexual development, enlarged penis), it is necessary to stop the drug. Testosterones may cause or aggravate acne. Scrupulous skin care and other antiacne treatment may be necessary, especially in adolescent boys.
Use in Older Adults
In older adults with hypertension and other cardiovascular disorders, the sodium and water retention associated with testosterone may aggravate these conditions. Also, in men, the drugs may increase prostate size and interfere with urination, increase risk of prostatic cancer, and cause excessive sexual stimulation and priapism.
Adverse Effects
Common adverse effects from testosterone include acne, change in sex drive, hair loss, headache, bitter taste or mouth irritation, or gum tenderness. Additionally, hypercalcemia, jaundice, and edema may occur. Virilizing or masculinizing effects may vary. In adult men with adequate secretion of testosterone, adverse effects include priapism, increased sexual desire, reduced sperm count, and prostate enlargement. In prepubertal boys, adverse effects may involve premature development of sex organs and secondary sexual characteristics, such as enlargement of the penis and pubic hair. Priapism may occur. In women, adverse effects include hirsutism, deepening of the voice, and menstrual irregularities.
Contraindications
Contraindications to testosterone include pregnancy (because of possible masculinizing effects on a female fetus), preexisting liver disease, and disorders of the prostate. (Men with an enlarged prostate may have additional enlargement, and men with prostate cancer may experience tumor growth.)
Nursing Implications
Preventing Interactions
Some medications and herbs interact with testosterone, decreasing its effects (Box 45.2). Androgens may increase effects of cyclosporine and warfarin, apparently by slowing their metabolism and increasing their concentrations in the blood. If possible, people should avoid using these combinations. If they are necessary, the nurse monitors serum creatinine and cyclosporine levels (for cyclosporine) and prothrombin time or international normalized ratio (INR) (for warfarin). Androgens also increase the effects of sulfonylureas. If possible, people should avoid using sulfonylureas concurrently.
BOX 45.2  Drug Interactions: Testosterone
Drug Interactions: Testosterone
Drugs That Decrease the Effects of Testosterone
 Barbiturates
Barbiturates
Increase enzyme induction and rate of metabolism
 Calcitonin
Calcitonin
Decreases calcium retention, thus antagonizing the calcium-retaining effects of androgens
Administering the Medication
Naturally occurring androgens are given by injection because they are metabolized rapidly by the liver if given orally. Some esters of testosterone have been modified to slow the rate of metabolism and thus prolong action. For example, intramuscular (IM) testosterone cypionate and testosterone enanthate have slow onsets of action and last 2 to 4 weeks. As a result of first-pass metabolism, doses as high as 400 mg/d may be needed to produce adequate blood levels for full replacement therapy.
Several transdermal formulations of testosterone are available. They have a rapid onset of action and last approximately 24 hours. A topical gel (a 10g dose delivers 100 mg) produces normal serum testosterone levels within 4 hours after application, and absorption of testosterone into the blood continues for 24 hours. Steady-state serum concentrations occur by the second or third day of use. When the gel is discontinued, serum testosterone levels remain in the normal range for 24 to 48 hours but decrease to pretreatment levels within about 5 days.
Assessing for Therapeutic and Adverse Effects
The nurse assesses for return of sex hormone levels, development of masculine characteristics in boys, and return of libido in adult males.
The nurse should assess for adverse effects, including hypersensitivity reaction, changes in sexual drive or masculinization, hair loss, changes in taste, oral irritation, and/or gum tenderness. The nurse also monitors serum calcium and observes for signs of hypercalcemia (e.g., kidney stones, polyuria, abdominal pain, nausea, vomiting, depression).
Patient Teaching
Box 45.3 presents patient teaching information for androgens.
BOX 45.3  Patient Teaching Guidelines for Androgens
Patient Teaching Guidelines for Androgens



