Differentiate between type 1 and type 2 diabetes mellitus.
 Understand the major effects of endogenous insulin on body tissues.
Understand the major effects of endogenous insulin on body tissues.
 Identify the clinical manifestations of type 1 and type 2 diabetes mellitus.
Identify the clinical manifestations of type 1 and type 2 diabetes mellitus.
 Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the insulins.
Identify the prototype and describe the action, use, adverse effects, contraindications, and nursing implications for the insulins.
 Discuss characteristics of the various types of insulins and insulin analogs.
Discuss characteristics of the various types of insulins and insulin analogs.
 Identify the various prototypes and describe the actions, uses, adverse effects, contraindications, and nursing implications for the oral antidiabetic drugs.
Identify the various prototypes and describe the actions, uses, adverse effects, contraindications, and nursing implications for the oral antidiabetic drugs.
 Identify the different prototypes and describe the actions, uses, adverse effects, contraindications, and nursing implications for the amylin analogs, incretin mimetics, and dipeptidyl peptidase-4 (DPP-4) inhibitors.
Identify the different prototypes and describe the actions, uses, adverse effects, contraindications, and nursing implications for the amylin analogs, incretin mimetics, and dipeptidyl peptidase-4 (DPP-4) inhibitors.
 Implement the nursing process in the care of patients receiving medications for the treatment of diabetes mellitus.
Implement the nursing process in the care of patients receiving medications for the treatment of diabetes mellitus.
• Explain the benefits of maintaining glycemic control in preventing complications of diabetes.
• Assist patients or caregivers in learning how to manage diabetes care, including administration of medication agents used to manage diabetes.
• Assess and monitor patients’ adherence to prescribed management strategies.
Clinical Application Case Study
Alfred Smith, a 56-year-old librarian, visits his physician because he has been feeling more tired and weak than usual for the past 2 months. On questioning, the physician learns that Mr. Smith often gets up in the middle of the night to urinate and drinks a glass of water. He also has numbness and tingling in his hands and feet, as well as blurred vision, which affects his job. Laboratory results show an impaired fasting glucose of 160 mg/dL and urinalysis of 4+ glucose. His lipid panel also shows total cholesterol of 240 mg/dL, highdensity lipoprotein cholesterol of 22 mg/dL, and triglycerides of 400 mg/dL. Mr. Smith receives a diagnosis of type 2 diabetes, and his physician places him on glyburide (DiaBeta) 2.5 mg by mouth with breakfast daily. He also receives information about a diet and exercise routine to help control his diabetes.
KEY TERMS
Blood glucose level: blood sugar level in the body
Blood glucose meter: device that measures how much glucose is in the blood
Diabetes mellitus: chronic disease characterized by disordered metabolism of carbohydrates, fats, and protein, and hyperglycemia, due to a deficiency in the amount on action of insulin; the three main forms of diabetes are type 1, type 2, and gestational diabetes
Glucagon: pancreatic hormone that raises blood glucose levels by stimulating the liver to convert glycogen into glucose; it opposes insulin
Gluconeogenesis: formation of glucose from noncarbohydrate sources such as fats and amino acids
Glucose: sugar in the blood; major stimulus of insulin secretion
Impaired fasting glucose: fasting blood glucose level between 100 mg/dL to 125 mg/dL; also referred to as prediabetes
Insulin: protein hormone secreted by beta cells in the pancreas; facilitates glucose utilization by cells. Absence of insulin results in diabetes mellitus
Insulin pump: wearable delivery system for continuous subcutaneous insulin infusion; the insulin dosage is programmed into the pump, and the appropriate amount of insulin is injected through a needle into the adipose tissue
Ketoacidosis: metabolic acidosis due to accumulation of ketone bodies formed by the breakdown of fatty acids and amino acids for energy in the absence of insulin
Lancet: sharp instrument used to prick the finger for a blood test
Introduction
Diabetes mellitus is characterized as a disease of insulin availability that eventually results in high blood glucose concentrations. Treatment of diabetes mellitus includes drugs to maintain blood glucose levels within the normal range and prevent complications. Insulin and oral hypoglycemic agents are two of the several types of medications used to lower blood glucose in diabetes mellitus. New drug therapies include amylin analogs, incretin mimetics, and dipeptidyl peptidase-4 (DPP-4) inhibitors. It is important that nurses understand the characteristic of diabetes mellitus and the clinical use of insulin, oral hypoglycemic medications, and the newer drugs. They also need to be familiar with the effects of dietary herbal supplements on blood glucose levels.
Overview of Diabetes
Metabolic problems occur early in people with diabetes mellitus and are related to changes in the metabolism of carbohydrate, fat, and protein. A major clinical manifestation of disordered metabolism is hyperglycemia, or fasting blood glucose levels exceeding 126 mg/dL. A person with a fasting blood glucose level between 100 and 125 mg/dL is said to have impaired fasting glucose (IFG), or prediabetes. Indicative blood glucose levels for most people diagnosed with diabetes are approximately 80 to 120 mg/dL before a meal, 180 mg/dL or less after a meal, and between 100 and 140 mg/dL at bedtime.
Vascular problems include atherosclerosis throughout the body. Macrovascular (moderate and large vessels) clinical manifestations include hypertension, myocardial infarction, stroke, and peripheral vascular disease. Changes in small blood vessels (microvasculature) especially affect the retina and kidney, resulting in retinopathy, blindness, and nephropathy.
Etiology
The two major classifications of diabetes mellitus are type 1 and type 2. Although both are characterized by hyperglycemia, they differ in onset, course, pathology, and treatment. Other types of diabetes may be induced by disease processes such as slowly progressing autoimmune disorders, certain drugs, and pregnancy (see Chap. 6).
Type 1 Diabetes
Type 1 diabetes, a common chronic disorder of childhood, results from an autoimmune disorder that destroys pancreatic beta cells. Although it may occur at any age, it usually appears after 4 years of age and peaks in incidence at 10 to 12 years for girls and 12 to 14 years for boys. A subtype of type 1 diabetes, latent autoimmune diabetes of the adult, begins in adulthood. Symptoms of traditional type 1 diabetes usually develop when 10% to 20% of functioning beta cells remain, but they may occur at any time if acute illness or stress increases the body’s demand for insulin (a protein hormone secreted by the beta cells) beyond the capacity of the remaining beta cells to secrete insulin. Eventually, destruction of all beta cells occurs, resulting in no insulin production.
Type 1 diabetes usually has a sudden onset; produces severe symptoms; is difficult to control; produces a high incidence of complications, such as diabetic ketoacidosis (DKA) and renal failure; and requires administration of exogenous insulin. About 10% of people with diabetes have type 1 disease.
Type 2 Diabetes
Type 2 diabetes is characterized by hyperglycemia and insulin resistance. The hyperglycemia results from increased production of glucose by the liver and decreased uptake of glucose in liver, muscle, and fat cells. Insulin resistance means that higher-than-usual concentrations of insulin are required. Thus, insulin is present but unable to work effectively (i.e., inhibits hepatic production of glucose and causes glucose to move from the bloodstream into liver, muscle, and fat cells). Most insulin resistance is attributed to impaired insulin action at the cellular level, possibly related to postreceptor, intracellular mechanisms.
Historically, the onset of type 2 diabetes occurred after 40 years of age. More recently, however, type 2 diabetes is increasing in prevalence among children and adolescents. Compared with type 1, it usually has a gradual onset, produces less severe symptoms initially, is easier to control, causes less DKA and renal failure but more myocardial infarctions and strokes, and does not necessarily require exogenous insulin because endogenous insulin is still produced. About 90% of people with diabetes have type 2 disease; 20% to 30% of them require exogenous insulin at some point in their lives.
Type 2 is a heterogeneous disease, and etiology probably involves multiple factors such as a genetic predisposition and environmental factors. Obesity is a major cause. With obesity and chronic ingestion of excess calories, along with a sedentary lifestyle, more insulin is required. The increased need leads to prolonged stimulation and eventual “fatigue” of pancreatic beta cells. As a result, the cells become less responsive to elevated blood glucose levels and less able to produce enough insulin to meet metabolic needs. Thus, insulin is secreted but is inadequate or ineffective, especially when insulin demand is increased by obesity, pregnancy, aging, or other factors.
A risk factor for the development of type 2 diabetes is the presence of metabolic syndrome. This syndrome is characterized by a group of risk factors, including abdominal obesity (excessive fat tissue in and around the abdomen), hypertriglyceridemia, low high-density lipoprotein (HDL) cholesterol, hypertension, and/or IFG. People with metabolic syndrome have an increased risk of coronary heart disease and other diseases related to plaque buildup in artery walls (e.g., stroke and peripheral vascular disease) and type 2 diabetes. Metabolic syndrome has become increasingly common in the United States. Estimates indicate that more than 50 million Americans have it.
In the United States, African Americans, Hispanics, Native Americans, Native Alaskans, and some Asian Americans and Pacific Islanders are at high risk for type 2 diabetes. Prevalence rates are about 13.3% in African Americans and 13.9% in Hispanic Americans and 12.8% in American Indians and Alaska Natives compared with 8.7% in Caucasians. Undiagnosed diabetes is reportedly common in Mexican Americans.
Pathophysiology
Endogenous Insulin
Beta cells in the pancreas secrete insulin. The average adult pancreas secretes 40 to 60 units of insulin daily. This includes a basal amount of 1 to 2 units/h and additional amounts (4–6 units/h) after meals or when the blood glucose level exceeds 100 mg/dL. In a fasting state, serum insulin levels are low, and the body uses stored glucose and amino acids for energy needs of tissues that require glucose. After a meal, serum insulin levels increase and peak in a few minutes, then decrease to baseline levels in 2 to 3 hours.
Amylin, a pancreatic hormone secreted with insulin, delays gastric emptying, increases satiety, and suppresses glucagon (hormone that raises blood glucose levels by stimulating the liver to convert glycogen into glucose) secretion, thus complementing the effects of insulin on blood glucose. A synthetic form of amylin, pramlintide, has been developed to assist with glucose control in patients with diabetes (see later discussion).
Insulin is secreted into the portal circulation and transported to the liver, where about half is used or degraded. The other half reaches the systemic circulation, where it circulates mainly in an unbound form and is transported to body cells.
At the cellular level (Fig. 39.1), insulin binds with and activates receptors on the cell membranes of about 80% of body cells. Liver, muscle, and fat cells have many insulin receptors and are primary tissues for insulin action. After insulin–receptor binding occurs, cell membranes become highly permeable to glucose and allow rapid entry of glucose into the cells. The cell membranes also become more permeable to amino acids, fatty acids, and electrolytes such as potassium, magnesium, and phosphate ions. Cellular metabolism is altered by the movement of these substances into the cells, by activation of some enzymes and inactivation of others, by movement of proteins between intracellular compartments, by changes in the amounts of proteins produced, and perhaps by other mechanisms. Overall, the changes in cellular metabolism stimulate anabolic effects (e.g., utilization and storage of glucose, amino acids, and fatty acids) and inhibit catabolic processes (e.g., breakdown of glycogen, fat, and protein). After binding to insulin and entering the cell, receptors may be degraded or recycled back to the cell surface.
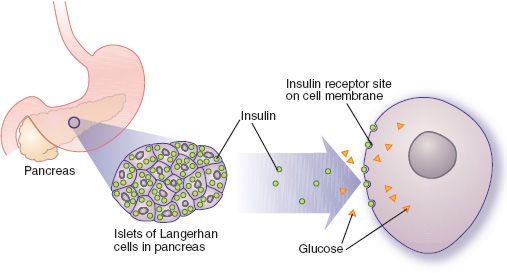
Figure 39.1 Normal glucose metabolism. After insulin binds with receptors on the cell membrane, glucose can move into the cell, promoting cellular metabolism and energy production.
Insulin is cleared from circulating blood in 10 to 15 minutes because of rapid binding to peripheral tissues or metabolic breakdown. The insulin that does not combine with receptors is metabolized in the liver, kidneys, plasma, and muscles. In the kidneys, insulin is filtered by the glomeruli and reabsorbed by the tubules, which also degrade it. Severe renal impairment slows the clearance of insulin from the blood.
Insulin plays a major role in metabolism of carbohydrate, fat, and protein (Box 39.1). Food particles are broken down into molecules of glucose, lipids, and amino acids, respectively. The molecules enter the cells and are converted to energy for cellular activities. The energy can be used immediately or converted to storage forms for later use. When carrying out its metabolic functions, the overall effect of insulin is to lower blood glucose levels, primarily by the following mechanisms:
BOX 39.1 Effects of Insulin on Metabolism
Carbohydrate Metabolism
 Insulin increases glucose transport into the liver, skeletal muscle, adipose tissue, the heart, and some smooth muscle organs (such as the uterus); it must be present for muscle and fat tissues to use glucose for energy.
Insulin increases glucose transport into the liver, skeletal muscle, adipose tissue, the heart, and some smooth muscle organs (such as the uterus); it must be present for muscle and fat tissues to use glucose for energy.
 Insulin regulates glucose metabolism to produce energy for cellular functions. If excess glucose is present after this need is met, it is converted to glycogen and stored for future energy needs or converted to fat and stored. The excess glucose transported to liver cells is converted to fat only after glycogen stores are saturated. When insulin is absent or blood glucose levels are low, these stored forms of glucose can be reconverted. The liver is especially important in restoring blood sugar levels by breaking down glycogen or by forming new glucose.
Insulin regulates glucose metabolism to produce energy for cellular functions. If excess glucose is present after this need is met, it is converted to glycogen and stored for future energy needs or converted to fat and stored. The excess glucose transported to liver cells is converted to fat only after glycogen stores are saturated. When insulin is absent or blood glucose levels are low, these stored forms of glucose can be reconverted. The liver is especially important in restoring blood sugar levels by breaking down glycogen or by forming new glucose.
Fat Metabolism
 Insulin promotes transport of glucose into fat cells, where it is broken down. One of the breakdown products is alpha-glycerophosphate, which combines with fatty acids to form triglycerides. This is the mechanism by which insulin promotes fat storage.
Insulin promotes transport of glucose into fat cells, where it is broken down. One of the breakdown products is alpha-glycerophosphate, which combines with fatty acids to form triglycerides. This is the mechanism by which insulin promotes fat storage.
 When insulin is lacking, fat is released into the bloodstream as free fatty acids. Blood concentrations of triglycerides, cholesterol, and phospholipids are also increased. The high blood lipid concentration probably accounts for the atherosclerosis that tends to develop early and progress more rapidly in people with diabetes mellitus. Also, when more fatty acids are released than the body can use as fuel, some fatty acids are converted into ketones. Excessive amounts of ketones produce acidosis and coma.
When insulin is lacking, fat is released into the bloodstream as free fatty acids. Blood concentrations of triglycerides, cholesterol, and phospholipids are also increased. The high blood lipid concentration probably accounts for the atherosclerosis that tends to develop early and progress more rapidly in people with diabetes mellitus. Also, when more fatty acids are released than the body can use as fuel, some fatty acids are converted into ketones. Excessive amounts of ketones produce acidosis and coma.
Protein Metabolism
 Insulin increases the total amount of body protein by increasing transport of amino acids into cells and synthesis of protein within the cells. The basic mechanism of these effects is unknown.
Insulin increases the total amount of body protein by increasing transport of amino acids into cells and synthesis of protein within the cells. The basic mechanism of these effects is unknown.
 Insulin potentiates the effects of growth hormone.
Insulin potentiates the effects of growth hormone.
 Lack of insulin causes protein breakdown into amino acids, which are released into the bloodstream and transported to the liver for energy or gluconeogenesis. The lost proteins are not replaced by synthesis of new proteins, and protein wasting causes abnormal functioning of many body organs, severe weakness, and weight loss.
Lack of insulin causes protein breakdown into amino acids, which are released into the bloodstream and transported to the liver for energy or gluconeogenesis. The lost proteins are not replaced by synthesis of new proteins, and protein wasting causes abnormal functioning of many body organs, severe weakness, and weight loss.
• In the liver, insulin acts to decrease breakdown of glycogen (glycogenolysis), form new glucose from fatty acids and amino acids (gluconeogenesis), and form ketone bodies (ketogenesis). At the same time, it acts to increase synthesis and storage of glycogen and fatty acids.
• In adipose tissue, insulin acts to decrease breakdown of fat (lipolysis) and to increase production of glycerol and fatty acids.
• In muscle tissue, insulin acts to decrease protein breakdown and amino acid output and to increase amino acid uptake, protein synthesis, and glycogen synthesis.
Regulation of Insulin Secretion
Insulin decreases blood sugar and regulates the amount of glucose available for cellular metabolism and energy needs, during both fasting and feeding. Insulin secretion involves coordination of various nutrients, hormones, the autonomic nervous system, and other factors.
Glucose is the major stimulus of insulin secretion; others include amino acids, fatty acids, ketone bodies (which are acidotic substances in themselves), and stimulation of beta2-adrenergic receptors or vagal nerves. Oral glucose is more effective than intravenous (IV) glucose because glucose or food in the digestive tract stimulates vagal activity and induces the release of gastrointestinal (GI) hormones called incretins. Incretin hormones stimulate the release of insulin when glucose levels are normal or elevated. In addition, incretin hormones reduce glucagon production and delay gastric emptying. Two incretin hormones have been identified: glucose-dependent insulinotropic peptide and glucagon-like peptide-1 (GLP-1). GLP-1 also appears to improve insulin sensitivity and may increase the formation of new beta cells in the pancreas. The therapeutic uses of incretin hormones have recently been investigated, and this has led to the development of new drugs to treat diabetes mellitus.
Several hormones raise blood glucose levels and stimulate insulin secretion, including cortisol, glucagon, growth hormone, epinephrine, estrogen, and progesterone. Excessive, prolonged, endogenous secretion or administration of pharmacologic preparations of these hormones can exhaust the ability of pancreatic beta cells to produce insulin and thereby cause or aggravate diabetes mellitus.
Factors that inhibit insulin secretion include stimulation of pancreatic alpha2-adrenergic receptors and stress conditions such as hypoxia, hypothermia, surgery, or severe burns.
Clinical Manifestations
Most signs and symptoms stem from a lack of effective insulin and the subsequent metabolic abnormalities. The incidence and severity depend on the amount of effective insulin, and conditions such as infection, rapid growth, pregnancy, or other factors may increase demand for insulin. Most early symptoms result from disordered carbohydrate metabolism, which causes excess glucose to accumulate in the blood (hyperglycemia). Hyperglycemia produces glucosuria, which, in turn, produces polydipsia, polyuria, dehydration, and polyphagia.
Glucosuria usually appears when the blood glucose level is approximately twice the normal value and the kidneys receive more glucose than can be reabsorbed. However, renal threshold varies, and the amount of glucose lost in the urine does not accurately reflect blood glucose. In children, glucose tends to appear in urine at much lower or even normal blood glucose levels. In older people, the kidneys may be less able to excrete excess glucose from the blood. As a result, blood glucose levels may be high with little or no glucose in the urine.
When large amounts of glucose are present, water is pulled into the renal tubule. This results in a greatly increased urine output (polyuria). The excessive loss of fluid in urine leads to increased thirst (polydipsia) and, if fluid intake is inadequate, to dehydration. Dehydration also occurs because high blood glucose levels increase osmotic pressure in the bloodstream and fluid is pulled out of the cells in the body’s attempt to regain homeostasis. Polyphagia (increased appetite) occurs because the body cannot use ingested foods. People with uncontrolled diabetes lose weight because of abnormal metabolism.
Complications of diabetes mellitus are common and potentially disabling or life threatening. Diabetes is a leading cause of myocardial infarction, stroke, blindness, leg amputation, and kidney failure. These complications result from hyperglycemia and other metabolic abnormalities that accompany a lack of effective insulin. The metabolic abnormalities associated with hyperglycemia can cause early, acute complications, such as DKA or hyperosmolar hyperglycemic nonketotic coma (HHNC; Box 39.2). Eventually, metabolic abnormalities lead to damage in blood vessels and other body tissues. For example, atherosclerosis develops earlier, progresses more rapidly, and becomes more severe in people with diabetes. Microvascular changes lead to nephropathy, retinopathy, and peripheral neuropathy. Other complications include musculoskeletal disorders, increased numbers and severity of infections, and complications of pregnancy.
BOX 39.2 Acute Complications of Diabetes Mellitus
Diabetic Ketoacidosis (DKA)
This life-threatening complication occurs with severe insulin deficiency. In the absence of insulin, glucose cannot be used by body cells for energy, and fat is mobilized from adipose tissue to furnish a fuel source. The mobilized fat circulates in the bloodstream, from which it is extracted by the liver and broken down into glycerol and fatty acids. The fatty acids are further changed in the liver to ketones (e.g., acetoacetic acid, acetone), which then enter the bloodstream and are circulated to body cells for metabolic conversion to energy, carbon dioxide, and water.
The ketones are produced more rapidly than body cells can use them, and their accumulation produces acidemia (a drop in blood pH and an increase in blood hydrogen ions). The body attempts to buffer the acidic hydrogen ions by exchanging them for intracellular potassium ions. Hydrogen ions enter body cells, and potassium ions leave the cells to be excreted in the urine. Another attempt to remove excess acid involves the lungs. Deep, labored respirations, called Kussmaul’s respirations, eliminate more carbon dioxide and prevent formation of carbonic acid. A third attempt to regain homeostasis involves the kidneys, which excrete some of the ketones, thereby producing acetone in the urine.
Two major causes of DKA are omission of insulin and illnesses such as infection, trauma, myocardial infarction, or stroke. DKA worsens as the compensatory mechanisms fail. Clinical signs and symptoms become progressively more severe. Early ones include blurred vision, anorexia, nausea and vomiting, thirst, and polyuria. Later ones include drowsiness, which progresses to stupor and coma; Kussmaul’s respirations; dehydration and other signs of fluid and electrolyte imbalances; and decreased blood pressure, increased pulse, and other signs of shock.
Insulin therapy is a major part of any treatment for DKA. Patients with DKA have a deficiency in the total amount of insulin in the body and a resistance to the action of the insulin that is available, probably due to acidosis, hyperosmolality, infection, and other factors. To be effective, insulin therapy must be individualized according to frequent measurements of blood glucose. Low doses, given by continuous intravenous (IV) infusion, are preferred in most circumstances so that the brain has time to equilibrate and account for fluid shifts.
Additional measures include identification and treatment of conditions that precipitate DKA, administration of IV fluids to correct hyperosmolality and dehydration, administration of potassium supplements to restore and maintain normal serum potassium levels, and administration of sodium bicarbonate to correct metabolic acidosis. Infection is one of the most common causes of DKA. If no obvious source of infection is identified, cultures of blood, urine, and throat swabs are recommended. When infection is identified, antimicrobial drug therapy may be indicated.
Hyperosmolar Hyperglycemic Nonketotic Coma (HHNC)
HHNC is another type of diabetic coma that is potentially life threatening. It is relatively rare and carries a high mortality rate. The term hyperosmolar refers to an excessive amount of glucose, electrolytes, and other solutes in the blood in relation to the amount of water.
Like DKA, HHNC is characterized by hyperglycemia, which leads to osmotic diuresis and resultant thirst, polyuria, dehydration, and electrolyte losses, as well as neurologic signs ranging from drowsiness to stupor to coma. Additional clinical problems may include hypovolemic shock, thrombosis, renal problems, or stroke. In contrast with DKA, hyperosmolar coma occurs in people with previously unknown or mild diabetes, usually after an illness; occurs in hyperglycemic conditions other than diabetes (e.g., severe burns, corticosteroid drug therapy); and does not cause ketosis.
Treatment of HHNC is similar to that of DKA in that insulin, IV fluids, and potassium supplements are major components. Regular insulin is given by continuous IV infusion, and dosage is individualized according to frequent measurements of blood glucose levels. IV fluids are given to correct the profound dehydration and hyperosmolality, and potassium is given IV to replace the large amounts lost in urine during a hyperglycemic state.
Initiation of Intensive Diabetes Control in the First 15 Years After Diagnosis Reduces Cardiovascular Events
by AMERICAN DIABETES ASSOCIATION
Retrieved May 30, 2011, from http://www.diabetes.org/for-media/2009/factors-affecting-2009.html
Patients with diabetes mellitus are at an increased risk of developing macrovascular and microvascular complications secondary to the nature of the disease and poor glycemic control. In this study, researchers found that initiation of intensive control in the first 15 years after a diagnosis of diabetes reduced the risk of cardiovascular events, including mortality, but initiation 16 to 20 years after diagnosis yielded no such benefit. Further, initiation of intensive control 20 or more years after diagnosis actually increased the risk of cardiovascular events. Severe hypoglycemia was mentioned as a serious risk factor in tight glycemic control. Patients with severe hypoglycemic events were undoubtedly at an increased risk for cardiovascular events regardless of the degree of glycemic control.
IMPLICATIONS FOR NURSING PRACTICE: When providing care to a patient with diabetes mellitus, it is important to assess the patient’s knowledge of the disease process and individual treatment plan. The nurse should teach the patient to collaborate closely with all involved members of the health care personnel directly involved in the care and management of the treatment plan. The nurse should encourage the patient to maintain a diet and exercise regimen to achieve tight glycemic control and provide education when needed.
Drug Therapy
Medications used in the treatment of diabetes mellitus depend on the type of diabetes and degree of glycemic control. Health care providers use many medications to control diabetes (Table 39.1). Insulin is the prototype drug for treatment of type 1 diabetes. Several different classes of other drugs are also available for the treatment of type 2 diabetes (Fig. 39.2). Patients with diabetes may be using herbal supplements, and nurses should be aware that some of these substances affect blood glucose levels (Box 39.3).
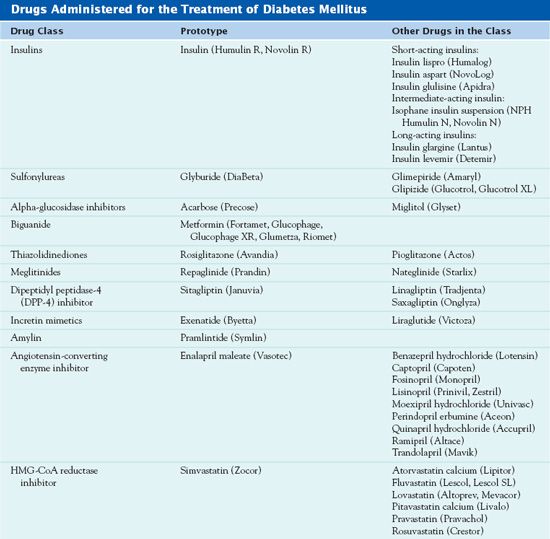
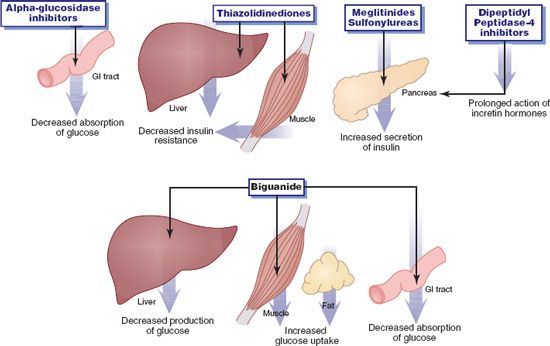
Figure 39.2 Actions of oral antidiabetic drugs. The drugs lower blood sugar by decreasing absorption or production of glucose, by increasing secretion of insulin, or by increasing the effectiveness of available insulin (decreasing insulin resistance).
BOX 39.3  Herbs That Increase the Risk of Hypoglycemia When Used With Antidiabetic Drugs
Herbs That Increase the Risk of Hypoglycemia When Used With Antidiabetic Drugs
 Alfalfa
Alfalfa
 Aloe
Aloe
 Bilberry
Bilberry
 Bitter melon
Bitter melon
 Burdock
Burdock
 Celery
Celery
 Coriander
Coriander
 Damiana
Damiana
 Dandelion root
Dandelion root
 Fenugreek
Fenugreek
 Garcinia
Garcinia
 Garlic Ginseng
Garlic Ginseng
 Gymnema
Gymnema
 Juniper
Juniper
 Karela
Karela
 Marshmallow
Marshmallow
 Stinging nettle
Stinging nettle
Clinical Application 39-1
 What additional condition does Mr. Smith have?
What additional condition does Mr. Smith have?
 What are the complications related to this diagnosis?
What are the complications related to this diagnosis?
NCLEX Success
1. A patient presents with a blood pressure of 162/88 mm Hg, heart rate of 100 bpm, triglycerides of 378 mg/dL, and HDL of 25 mg/dL. Which of the following are characteristics of metabolic syndrome? (Select all that apply.)
A. blood pressure of 162/88 mm Hg
B. heart rate of 100 bpm
C. triglycerides of 378 mg/dL
D. HDL of 25 mg/dL
2. A patient who has just arrived at the emergency department may be suffering from diabetic ketoacidosis. Which of the following would confirm the diagnosis?
A. elevated serum potassium
B. increased respiratory rate
C. increased pH
D. elevated blood glucose level and low plasma bicarbonate level
Insulins
Insulin in its various forms is the only effective drug treatment for type 1 diabetes, where pancreatic beta cells are unable to secrete endogenous insulin, and metabolism is severely impaired.  Regular insulin (Humulin R, Novolin R) is the prototype. Insulin is also necessary in patients with type 2 diabetes who cannot control their disease with diet, weight control, and oral agents. Any person with diabetes may need insulin during times of stress, such as illness, infection, or surgery. Other uses of insulin include control of diabetes induced by chronic pancreatitis, surgical excision of pancreatic tissue, hormones and other drugs, and pregnancy (gestational diabetes). In patients who do not have diabetes, health care providers use insulin to prevent or treat hyperglycemia induced by IV parenteral nutrition and to treat hyperkalemia. In hyperkalemia, an IV infusion of insulin and dextrose solution causes potassium to move from the blood into the cells; it does not eliminate potassium from the body.
Regular insulin (Humulin R, Novolin R) is the prototype. Insulin is also necessary in patients with type 2 diabetes who cannot control their disease with diet, weight control, and oral agents. Any person with diabetes may need insulin during times of stress, such as illness, infection, or surgery. Other uses of insulin include control of diabetes induced by chronic pancreatitis, surgical excision of pancreatic tissue, hormones and other drugs, and pregnancy (gestational diabetes). In patients who do not have diabetes, health care providers use insulin to prevent or treat hyperglycemia induced by IV parenteral nutrition and to treat hyperkalemia. In hyperkalemia, an IV infusion of insulin and dextrose solution causes potassium to move from the blood into the cells; it does not eliminate potassium from the body.
All insulin in the United States is human insulin. Pork and bovine insulins, which were more antigenic, are no longer manufactured in the United States. The name human insulin means that the synthetic product is identical to endogenous insulin (i.e., has the same number and sequence of amino acids).
Types of Insulin
Insulins differ in onset and duration of action. They are usually categorized as short, intermediate, or long acting. The synthesis of insulin analogs involves altering the type or sequence of amino acids in insulin molecules.
Short-acting insulins have a rapid onset (15 minutes or less) and a short duration of action (4-8 hours). Short-acting products include insulin lispro (Humalog), insulin aspart (NovoLog), and insulin glulisine (Apidra). Lispro, the first insulin analog to be marketed, is identical to human insulin except for the reversal of two amino acids (lysine and proline). It is absorbed more rapidly and has a shorter half-life after subcutaneous injection than regular (short-acting) human insulin. As a result, it is similar to physiologic insulin secretion after a meal, more effective at decreasing postprandial hyperglycemia, and less likely to cause hypoglycemia before the next meal. Injection just before a meal produces hypoglycemic effects similar to those of an injection of conventional regular insulin given 30 minutes before a meal. Aspart has an even more rapid onset and shorter duration of action. Glulisine, the newest short-acting insulin analog, has the shortest onset of action (5-10 minutes).
Intermediate-acting insulin preparations such as isophane (NPH) suspension possess zinc insulin crystals that have been modified by protamine in a neural buffer. The addition of zinc assists in slowing the absorption and thus prolongs the duration of action.
Long-acting insulin preparations include insulin glargine and insulin detemir. Health care providers use them to provide a basal amount of insulin through 24 hours, similar to normal, endogenous insulin secretion.
Several mixtures of an intermediate-and a short-acting insulin are available and in common use. U-100, the main insulin concentration in the United States, contains 100 units of insulin per milliliter of solution. Accurate measurement requires an orange-tipped syringe designed for use only with U-100 insulin.
After subcutaneous injection, insulin is absorbed most rapidly from the abdomen, followed by the upper arm, thigh, and buttocks. Absorption is delayed or decreased by injection into subcutaneous tissue with lipodystrophy or other lesions, by circulatory problems such as edema or hypotension, by insulin-binding antibodies (which develop after 2 or 3 months of insulin administration), and by injecting cold (i.e., refrigerated) insulin. Absorption may also be increased when administered in an extremity before the patient engages in a sport that requires use of the specific extremity (i.e., swimming, tennis, or jogging).
An inhaled insulin (Exubera) received U.S. Food and Drug Administration (FDA) approval in January 2006 for patients 18 years of age or older with type 1 or type 2 diabetes. However, by October 2007, the drug manufacturer made a decision to cease production of Exubera, citing that too few patients using inhaled insulin and the availability of other medications to lower blood sugar as reasons for discontinuing production. Currently, no FDA-approved inhaled insulins are available; however, several drug manufacturers are working to develop new forms of insulin delivery, including mouth sprays, insulin patches, and inhalers.
Table 39.2 gives information about insulins, including routes and dosages.
 TABLE 39.2
TABLE 39.2
DRUGS AT A GLANCE: Insulins

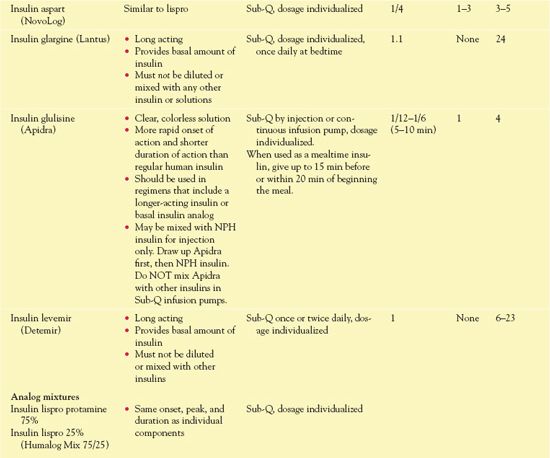
*All insulins are in U.S. Food and Drug Administration Pregnancy Category B.
†When mixing insulins, always draw up the clear insulin first, then add the cloudy insulin.
Choice of Insulin
When insulin therapy is indicated, the physician may choose from several preparations that vary in composition, onset, duration of action, and other characteristics. Some factors to be considered include the following:
• Regular insulin (insulin injection) has a rapid onset of action and can be given intravenously. Therefore, it is the insulin of choice during acute situations, such as DKA, severe infection or other illness, and surgical procedures.
• Isophane insulin (NPH) is often used for long-term insulin therapy. For many patients, a combination of NPH and short-acting insulin provides more consistent control of blood glucose levels. Although several regimens are used, a common one is a mixture of regular and NPH insulins administered before the morning and evening meals. A commercial mixture is more convenient and probably more accurate than a mixture prepared by a patient or caregiver, if the proportions of insulins are appropriate for the patient.
• Insulin lispro, aspart, or glulisine may be used instead of regular subcutaneous insulin in most situations, but safe use requires both health care providers and patients to be aware of differences. Regular insulin, insulin aspart, and insulin glulisine are also approved for use in external insulin pumps that administer a continuous subcutaneous infusion.
• Insulin glargine or insulin detemir may be used to provide a basal amount of insulin over 24 hours, with a short-acting insulin or short-acting insulin analog at meal times.
Pharmacokinetics
Regular insulin is rapidly absorbed after IV, intramuscular (IM), and subcutaneous administration. It is considered to be of short duration with a slow action. It is primarily metabolized in the liver, and a small amount is metabolized in the kidneys. Less than 2% of the drug is excreted in the urine.
Action
Insulin and its analogs (structurally similar chemicals) replace endogenous insulin, and this exogenous insulin has the same effects as the pancreatic hormone. The insulins lower blood glucose levels by increasing glucose uptake by body cells, especially skeletal muscle and fat cells, and by decreasing glucose production in the liver.
Use
Insulin is used to lower blood glucose, and the dosage must be individualized according to blood glucose levels. Blood glucose meters are devices that measure how much glucose is in the blood. A specially coated test strip containing a fresh sample of blood (obtained by pricking the skin, usually the finger or forearm, with a lancet) is inserted in the meter, which then measures the amount of glucose in the blood. The goal is to administer enough insulin to alleviate symptoms of hyperglycemia and to reestablish metabolic balance without causing hypoglycemia (Box 39.4). An initial dose of 0.5 to 1 units/kg/d may be started and then adjusted to maintain blood glucose levels (tested before meals and at bedtime) of 90 to 130 mg/dL in adults (100-200 mg/dL in children younger than 5 years of age). However, many factors influence blood glucose response to exogenous insulin and therefore influence insulin requirements.
BOX 39.4 Hypoglycemia: Characteristics and Management
Hypoglycemia may occur with insulin, meglitinides, oral sulfonylureas, amylin analogs (pramlintide [Symlin]), and incretin mimetics (exenatide [Byetta]). When hypoglycemia is suspected, the blood glucose level should be measured if possible, although signs and symptoms and the plasma glucose level at which they occur vary from person to person. Hypoglycemia is blood glucose below 60 to 70 mg/dL and is especially dangerous at approximately 40 mg/dL or below. Central nervous system effects may lead to accidental injury or permanent brain damage; cardiovascular effects may lead to cardiac dysrhythmias or myocardial infarction. Causes of hypoglycemia include:
 Intensive insulin therapy (i.e., continuous subcutaneous [Sub-Q] infusion or three or more injections daily) Omitting or delaying meals
Intensive insulin therapy (i.e., continuous subcutaneous [Sub-Q] infusion or three or more injections daily) Omitting or delaying meals
 An excessive or incorrect dose of insulin or an oral agent that causes hypoglycemia
An excessive or incorrect dose of insulin or an oral agent that causes hypoglycemia
 Altered sensitivity to insulin
Altered sensitivity to insulin
 Decreased clearance of insulin or an oral agent (e.g., with renal insufficiency)
Decreased clearance of insulin or an oral agent (e.g., with renal insufficiency)
 Decreased glucose intake
Decreased glucose intake
 Decreased production of glucose in the liver
Decreased production of glucose in the liver
 Giving an insulin injection via the intramuscular (IM) rather than the Sub-Q route
Giving an insulin injection via the intramuscular (IM) rather than the Sub-Q route
 Drug interactions that decrease blood glucose levels
Drug interactions that decrease blood glucose levels
 Increased physical exertion
Increased physical exertion
 Ethanol ingestion
Ethanol ingestion
Hormones That Raise Blood Sugar
Normally, when hypoglycemia occurs, several hormones (glucagon, epinephrine, growth hormone, and cortisol) work to restore and maintain blood glucose levels. Glucagon and epinephrine, the dominant counter-regulatory hormones, act rapidly because they are activated as soon as blood glucose levels start declining. Growth hormone and cortisol act more slowly, about 2 hours after hypoglycemia occurs.
People with diabetes who develop hypoglycemia may have impaired secretion of these hormones, especially those patients with type 1 diabetes. Decreased secretion of glucagon is often evident in patients who have had diabetes for 5 years or longer. Decreased secretion of epinephrine also occurs in people who have been treated with insulin for several years. Decreased epinephrine decreases tachycardia, a common sign of hypoglycemia, and may delay recognition and treatment.
The Conscious Patient
Treatment of hypoglycemic reactions consists of immediate administration of a rapidly absorbed carbohydrate. For the conscious patient who is able to swallow, the carbohydrate is given orally. Foods and fluids that provide approximately 15 g of carbohydrate include:
 Liquids or fruit juices
Liquids or fruit juices
 4 teaspoons of sugar
4 teaspoons of sugar
 Commercial glucose products (e.g., Glutose, B-D Glucose). These products must be swallowed to be effective.
Commercial glucose products (e.g., Glutose, B-D Glucose). These products must be swallowed to be effective.
 Symptoms usually subside within 15 to 20 minutes. If they do not subside, the patient should take another 10 to 15 g of oral carbohydrate.
Symptoms usually subside within 15 to 20 minutes. If they do not subside, the patient should take another 10 to 15 g of oral carbohydrate.
 If acarbose or miglitol has been taken with insulin or a sulfonylurea and a hypoglycemic reaction occurs, glucose (oral or intravenous [IV]) or glucagon must be given for treatment. Sucrose (table sugar) and other oral carbohydrates do not relieve hypoglycemia because the presence of acarbose or miglitol prevents their digestion and absorption from the gastrointestinal tract.
If acarbose or miglitol has been taken with insulin or a sulfonylurea and a hypoglycemic reaction occurs, glucose (oral or intravenous [IV]) or glucagon must be given for treatment. Sucrose (table sugar) and other oral carbohydrates do not relieve hypoglycemia because the presence of acarbose or miglitol prevents their digestion and absorption from the gastrointestinal tract.
The Unconscious Patient
Carbohydrate cannot be given orally. Therefore, the choices are parenteral glucose or glucagon.
 In the health care facility, administer 25% to 50% dextrose solution
In the health care facility, administer 25% to 50% dextrose solution
 In home or elsewhere, give Sub-Q or IM glucagon 0.5 to 1 mg if available, and there is someone to inject it.
In home or elsewhere, give Sub-Q or IM glucagon 0.5 to 1 mg if available, and there is someone to inject it.
 Glucagon is a pancreatic hormone that increases blood sugar by converting liver glycogen to glucose. It is effective only when liver glycogen is present. Some patients cannot respond to glucagon because glycogen stores are depleted by conditions such as starvation, adrenal insufficiency, or chronic hypoglycemia. The hyperglycemic effect of glucagon occurs more slowly than that of IV glucose and is of relatively brief duration. If the patient does not respond to one or two doses of glucagon within 20 minutes, IV glucose is indicated.
Glucagon is a pancreatic hormone that increases blood sugar by converting liver glycogen to glucose. It is effective only when liver glycogen is present. Some patients cannot respond to glucagon because glycogen stores are depleted by conditions such as starvation, adrenal insufficiency, or chronic hypoglycemia. The hyperglycemic effect of glucagon occurs more slowly than that of IV glucose and is of relatively brief duration. If the patient does not respond to one or two doses of glucagon within 20 minutes, IV glucose is indicated.
Avoid Overtreatment
Caution is necessary in the treatment of hypoglycemia. Although the main goal of treatment is to relieve hypoglycemia and restore the brain’s supply of glucose, a secondary goal is to avoid overtreatment and excessive hyperglycemia.
• Factors that increase insulin requirements include weight gain, increased caloric intake, pregnancy, decreased activity, acute infections, hyperadrenocorticism (Cushing’s disease), primary hyperparathyroidism, acromegaly, hypokalemia, and drugs such as corticosteroids, epinephrine, levothyroxine, and thiazide diuretics. Patients who are obese may require 2 units/kg/d because of resistance to insulin in peripheral tissues.
• Factors that decrease insulin requirements include weight reduction; decreased caloric intake; increased physical activity; development of renal insufficiency; stopping administration of corticosteroids, epinephrine, levothyroxine, and diuretics; hypothyroidism; hypopituitarism; recovery from hyperthyroidism; recovery from acute infections; and the “honeymoon period,” which may occur with type 1 diabetes.
• People who need less than 0.5 units/kg/d may produce some endogenous insulin, or their tissues may be more responsive to insulin because of exercise and good physical conditioning.
• In acute situations, dosage of regular insulin needs frequent adjustments based on measurements of blood glucose. When insulin is given intravenously in a continuous infusion, 20% to 30% binds to the IV fluid container and the infusion tubing.
• Dosage of insulin for long-term therapy is determined by blood glucose levels at various times of the day and is adjusted when indicated (e.g., because of illness or changes in physical activity). Titrating insulin dosage may be difficult and time consuming; it requires cooperation and collaboration between patients and health care providers.
• Insulin has been used successfully with all currently available types of oral agents (alpha-glucosidase inhibitors, biguanide, thiazolidinediones, meglitinides, and sulfonylureas).
Use in Children
Effective management of type 1 diabetes requires a consistent schedule of meals, snacks, blood glucose monitoring, insulin injections and dose adjustments, and exercise. Insulin is the only drug indicated for use as replacement therapy because affected children cannot produce insulin. It is essential that insulin injections be given three or four times per day. Rotation of injection sites is important in infants and young children because of the relatively small areas for injection at each anatomic site and to prevent lipodystrophy. Young children usually adjust to injections and blood glucose monitoring better when the parents express less anxiety about these vital procedures.
A healthful, varied diet, rich in whole grains, fruits, and vegetables and limited in simple sugars, is recommended. According to the American Diabetes Association (2006), medical nutrition therapy should be tailored to a person’s specific health issues and personal preferences to help maintain optimum health by controlling blood glucose levels, blood pressure, cholesterol, and other risk factors.
It is essential to synchronize food intake with insulin injections, and this coordination usually involves three meals and three snacks, all at regularly scheduled times. Such a schedule is difficult to maintain in children, but it is extremely important in promoting normal growth and development. A major factor in optimal treatment is a supportive family in which at least one member is thoroughly educated about the disease and its management. Less-than-optimal treatment can lead to stunted growth, delayed puberty, and early development of complications such as retinopathy, nephropathy, or neuropathy.
Infections and other illnesses may cause wide fluctuations in blood glucose levels and interfere with metabolic control. For example, viral infections cause hypoglycemia; others, especially chronic infections, may cause hyperglycemia and insulin resistance and may precipitate ketoacidosis. As a result, insulin requirements may vary widely during illness episodes and should be based on blood glucose and urine ketone levels. Hypoglycemia often develops in young children, partly because of anorexia and smaller glycogen reserves.
During illness, children are highly susceptible to dehydration, and an adequate fluid intake is very important. Many clinicians recommend sugar-containing liquids (e.g., regular sodas, clear juices, and regular gelatin desserts) if blood glucose values are lower than 250 mg/dL. When blood glucose values are above 250 mg/dL, children should receive diet soda, unsweetened tea, and other fluids without sugar.
Administration of insulin for infants and toddlers who weigh less than 10 kg or require less than 5 units of insulin per day can be difficult because small doses are difficult to measure in a U-100 syringe. Use of diluted insulin allows more accurate administration. The most common dilution strength is U-10 (10 units/mL), and a diluent is available from insulin manufacturers for this purpose. It is necessary to clearly label vials of diluted insulin and discard them after 1 month.
Avoiding hypoglycemia is a major goal in infants and young children because of potentially damaging effects on growth and development. There may be a delay in recognition of hypoglycemia because signs and symptoms are vague and children may be unable to communicate them to parents or caregivers. Most pediatric endocrinologists recommend maintaining blood glucose levels between 100 and 200 mg/dL to prevent hypoglycemia. It is important never to skip the bedtime snack and blood glucose measurement.
Signs and symptoms of hypoglycemia in older children are similar to those in adults (e.g., hunger, sweating, and tachycardia). In young children, hypoglycemia may be manifested by changes in behavior, including severe hunger, irritability, and lethargy. In addition, mental functioning may be impaired in all age groups, even with mild hypoglycemia. Any time, hypoglycemia is suspected; it is essential that blood glucose be tested.
Adolescents may resist adhering to their prescribed treatment regimens, and effective management may be especially difficult during this developmental period. Adolescents and young adults may delay, omit, or decrease dosage of insulin to fit in socially (e.g., by eating more, “sleeping in,” drinking alcohol) or to control their weight. Omitting or decreasing insulin dosage may lead to repeated episodes of ketoacidosis. Also, adolescent females may develop eating disorders.
Health care professionals are increasingly identifying type 2 diabetes in children. This trend is attributed mainly to obesity and inadequate exercise because most children with type 2 disease are seriously overweight and have poor eating habits. In addition, most are members of high-risk ethnic groups and have relatives with diabetes. Management involves exercise; weight loss; a more healthful diet; and in some cases, drug therapy, including insulin. It is also important to attend to treating comorbid conditions such as hypertension and hyperlipidemia.
Use in Older Adults
It is estimated that at least 20% of people older than 65 years of age have diabetes. General precautions for safe and effective use of oral hypoglycemic drugs apply to older adults, including close monitoring of blood glucose levels; however, control of cardiovascular risk factors may play a greater role in reducing morbidity and mortality in this population. In addition, older adults may have impaired vision, poor manual dexterity, or other problems that decrease their ability to perform needed tasks (e.g., self-administration of insulin, monitoring blood glucose levels, managing diet, and exercise).
Use in Patients With Renal Impairment
Frequent monitoring of blood glucose levels and dosage adjustments may be necessary. It is difficult to predict dosage needs because, on the one hand, less insulin is degraded by the kidneys (normally about 25%), and this may lead to higher blood levels of insulin if dosage is not reduced. On the other hand, muscles and possibly other tissues are less sensitive to insulin, and this insulin resistance may result in an increased blood glucose level if dosage is not increased. Overall, vigilance is required to prevent dangerous hypoglycemia, especially in patients whose renal function is unstable or worsening.
Use in Patients With Hepatic Impairment
Higher blood levels of insulin may occur in patients with hepatic impairment because less insulin may be degraded. Careful monitoring of blood glucose levels and insulin dosage reductions may be needed to prevent hypoglycemia.
Use in Patients With Critical Illness
Critically ill patients, with and without diabetes mellitus, often experience hyperglycemia associated with insulin resistance. Hyperglycemia may complicate the progress of critically ill patients, resulting in increased complications such as postoperative infections, poor recovery, and increased mortality. Tight glycemic control is a key factor in preventing complications and improving mortality in the patient in an intensive care unit.
Insulin is more likely to be used in critical illness than any of the oral agents. Reasons include the greater ability to titrate dosage needs in patients who are often debilitated and unstable, with varying degrees of cardiovascular, liver, and kidney impairment. One important consideration with IV insulin therapy is that 30% or more of a dose may adsorb into containers of IV fluid or infusion sets. In addition, many critically ill patients are unable to take oral drugs. Surgery may require use of insulin. Box 39.5 provides information about perioperative insulin therapy.
BOX 39.5 Perioperative Insulin Therapy
Patients with diabetes who undergo major surgery have increased risks of both surgical and diabetic complications. Risks associated with surgery and anesthesia are greater if diabetes is not well controlled and complications of diabetes (e.g., hypertension, nephropathy, vascular damage) are already evident. Hyperglycemia and poor metabolic control are associated with increased susceptibility to infection, poor wound healing, and fluid and electrolyte imbalances. Risks of diabetic complications are increased because the stress of surgery increases insulin requirements and may precipitate diabetic ketoacidosis. Metabolic responses to stress include increased secretion of catecholamines, cortisol, glucagon, and growth hormone, all of which increase blood glucose levels. In addition to hyperglycemia, protein breakdown, lipolysis, ketogenesis, and insulin resistance occur. The risk of hypoglycemia is also increased.
The goals of treatment are to avoid hypoglycemia, severe hyperglycemia, ketoacidosis, and fluid and electrolyte imbalances. Maintenance of blood glucose levels between 120 and 180 mg/dL during the perioperative period is desirable. In general, mild hyperglycemia (e.g., blood glucose levels between 150 and 250 mg/dL) is considered safer for the patient than hypoglycemia, which may go unrecognized during anesthesia and surgery. Because surgery is a stressful event that increases blood glucose levels and the body’s need for insulin, insulin therapy is usually required.
The goal of insulin therapy is to avoid ketosis from inadequate insulin and hypoglycemia from excessive insulin. Specific actions depend largely on the severity of diabetes and the type of surgical procedure. Diabetes should be well controlled before any type of surgery. Minor procedures usually require little change in the usual treatment program; major operations usually require a different medication regimen.



