Describe important elements in the physiology of hemostasis and thrombosis.
 Discuss possible consequences of blood clotting disorders.
Discuss possible consequences of blood clotting disorders.
 Compare and contrast heparin and warfarin in terms of indications for use, onset and duration of action, route of administration, blood tests used to monitor effects, and nursing process implications.
Compare and contrast heparin and warfarin in terms of indications for use, onset and duration of action, route of administration, blood tests used to monitor effects, and nursing process implications.
 Discuss antiplatelet agents in terms of indications for use and effects on blood coagulation.
Discuss antiplatelet agents in terms of indications for use and effects on blood coagulation.
 Discuss direct thrombin inhibitors in terms of indications and contraindications for use, routes of administration, and major adverse effects.
Discuss direct thrombin inhibitors in terms of indications and contraindications for use, routes of administration, and major adverse effects.
 Describe thrombolytic agents in terms of indications and contraindications for use, routes of administration, and major adverse effects.
Describe thrombolytic agents in terms of indications and contraindications for use, routes of administration, and major adverse effects.
 Identify the prototype drug for each drug class.
Identify the prototype drug for each drug class.
 Describe systemic hemostatic agents for treating overdoses of anticoagulant and thrombolytic drugs.
Describe systemic hemostatic agents for treating overdoses of anticoagulant and thrombolytic drugs.
 Understand how to use the nursing process in the care of patients receiving anticoagulant, antiplatelet, and thrombolytic agents.
Understand how to use the nursing process in the care of patients receiving anticoagulant, antiplatelet, and thrombolytic agents.
Clinical Application Case Study
Andrew Oliver is a 45-year-old man who works as a mental health counselor. He presents to a small community emergency department with an acute anterior ST elevation myocardial infarction. Within 20 minutes of arrival, he receives alteplase by continuous intravenous (IV) infusion over 3 hours. He also simultaneously receives an IV bolus of heparin and is started on a heparin drip. You are the nurse assigned to his care.
KEY TERMS
Anticoagulants: drugs that prevent formation of new clots and extension of clots already present; do not dissolve formed clots
Antiplatelets: drugs that prevent one or more steps in the prothrombotic activity of platelets
Embolus: object that migrates through the circulation until it lodges in a blood vessel, causing occlusion; may be a thrombus, fat, air, amniotic fluid, a bit of tissue, or bacterial debris
Essential thrombocythemia: chronic blood disorder characterized by the overproduction of platelets by megakaryocytes in the absence of another cause
Fibrinolysin: enzyme that breaks down the fibrin meshwork that stabilizes blood clots; also referred to as plasmin
Hemostasis: prevention or stoppage of blood loss from an injured blood vessel and is the process that maintains the integrity of the vascular compartment
Heparin-induced thrombocytopenia (HIT): immune-mediated prothrombotic reaction resulting in a decrease in platelet count associated with heparin administration in patients with detectable HIT antibodies
Plasmin: enzyme that breaks down the fibrin meshwork that stabilizes blood clots; also referred to as fibrinolysin
Plasminogen: inactive protein found in many body tissues and fluids
Prothrombotic reaction: adverse effect that leads to thrombogenesis
Thrombogenesis: formation of a blood clot
Thrombolysis: breakdown or dissolution of blood clots
Thrombolytics: drugs that dissolve blood clots
Thrombosis: formation of a blood clot
Thrombus: blood clot
Introduction
Anticoagulant, antiplatelet, and thrombolytic drugs are used in the prevention and management of thrombotic and thromboembolic disorders. Thrombogenesis (or thrombosis), the formation of blood clots, is a normal body defense mechanism to prevent blood loss. Thus, this process may be lifesaving when it occurs as a response to hemorrhage; however, it may be life-threatening when it occurs at other times, because the thrombus, or blood clot, can obstruct a blood vessel and block blood flow to tissues beyond the clot either at the site of clot formation or to another part of the body. To aid understanding of drug therapy for thrombotic disorders, normal hemostasis, endothelial functions in relation to blood clotting, platelet functions, blood coagulation, and characteristics of arterial and venous thrombosis are described.
Overview of Coagulation Disorders
Physiology
Hemostasis
Hemostasis is the process that maintains the integrity of the vascular compartment. It involves activation of several mechanisms, including vasoconstriction, formation of a platelet plug (a cluster of aggregated platelets), sequential activation of clotting factors in the blood (Fig. 7.1), and growth of fibrous tissue (fibrin) into the blood clot to make it more stable and to repair the tear (opening) in the damaged blood vessel. Overall, normal hemostasis is a complex process involving numerous interacting activators and inhibitors, including endothelial factors, platelets, and blood coagulation factors (Box 7.1).
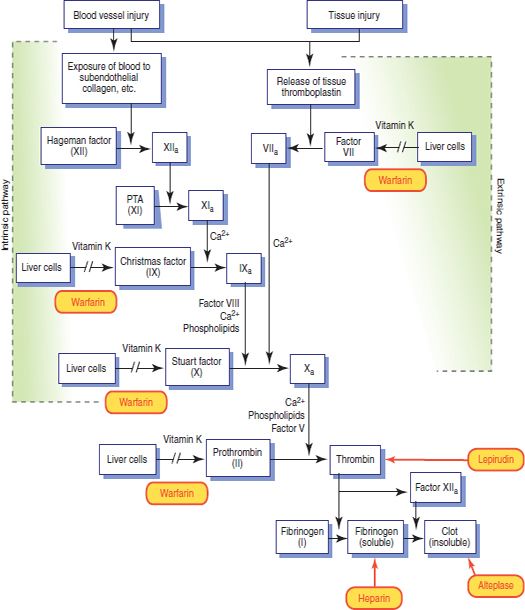
Figure 7.1 Details of the intrinsic and extrinsic clotting pathways. The sites of action of some of the drugs that can influence these processes are shown in red.
BOX 7.1 Hemostasis and Thrombosis
The blood vessels and blood normally maintain a balance between procoagulant and anticoagulant factors that favor anticoagulation and keeps the blood fluid. Injury to blood vessels and tissues causes complex reactions and interactions among vascular endothelial cells, platelets, and blood coagulation factors that shift the balance toward procoagulation and thrombosis.
Endothelial Cells
Normal endothelium helps to prevent thrombosis by producing anticoagulant factors, inhibiting platelet reactivity, and inhibiting activation of the coagulation cascade. However, endothelium promotes thrombosis if the blood vessel wall is damaged, function is altered, or when blood flow is altered or becomes static. After a blood clot is formed, the endothelium also induces its dissolution and restoration of blood flow.
Platelets
The only known function of platelets is hemostasis. Platelets (also called thrombocytes) are produced in the bone marrow and released into the bloodstream, where they circulate for approximately 7 to 10 days before they are removed by the spleen. They contain no nuclei and therefore cannot repair or replicate themselves.
The cell membrane of a platelet contains a coat of glycoproteins that prevents the platelet from adhering to normal endothelium but allows it to adhere to damaged areas of endothelium and collagen in the blood vessel wall. Breakdown of the cell membrane releases arachidonic acid (which can be metabolized to produce thromboxane A2) and allows leakage of platelet contents (e.g., thromboplastin and other clotting factors), which function to stop bleeding.
When platelets come in contact with a damaged vascular surface, they become activated and aggregate at a site of injury to help plug a hole in a torn blood vessel. Aggregated platelets release substances that recruit new platelets and stimulate additional aggregation. This activity helps the platelet plug become large enough to block blood flow out of a damaged blood vessel. If the opening is small, the platelet plug can stop blood loss. If the opening is large, a platelet plug and a blood clot are both required to stop the bleeding. Platelets usually disappear from a blood clot within 24 hours and are replaced by fibrin.
Blood Coagulation
The blood coagulation process represented in Figure 7.1 normally causes hemostasis within 1 to 2 minutes. It involves sequential activation of clotting factors that are normally present in blood and tissues as inactive precursors and formation of a meshwork of fibrin strands that cements blood components together to form a stable, dense clot. Major phases include release of thromboplastin by disintegrating platelets and damaged tissue; conversion of prothrombin to thrombin, which requires thromboplastin and calcium ions; and conversion of fibrinogen to fibrin by thrombin.
Blood coagulation results from activation of the intrinsic or extrinsic coagulation pathway. Both pathways, which are activated when blood passes out of a blood vessel, are needed for normal hemostasis. The intrinsic pathway occurs in the vascular system; the extrinsic pathway occurs in the tissues. Although the pathways are initially separate, the terminal steps (i.e., activation of factor X and thrombin- induced formation of fibrin) are the same leading to the formation of thrombin, which then activates fibrinogen to form fibrin, and the clot is complete.
Clot Lysis
When a blood clot is being formed, plasminogen, an inactive protein present in many body tissues and fluids, is bound to fibrin and becomes a component of the clot. After the outward blood flow is stopped and the tear in the blood vessel is repaired, plasminogen is activated by plasminogen activator (produced by endothelial cells or the coagulation cascade) to produce plasmin. Plasmin (also called fibrinolysin) is an enzyme that breaks down the fibrin meshwork that stabilizes the clot; this fibrinolytic or thrombolytic action dissolves the clot.
Etiology
Normally, thrombi are constantly being formed and dissolved, but the blood remains fluid and flow is not significantly obstructed. If the balance between thrombogenesis and thrombolysis, dissolution of blood clots, is upset, thrombotic or bleeding disorders ensue. Thrombosis may occur in both arteries and veins. Arterial thrombosis is usually associated with atherosclerotic plaque, hypertension, and turbulent blood flow. These conditions damage arterial endothelium and activate platelets to initiate the coagulation process. Arterial thrombi cause disease by obstructing blood flow. If the obstruction is incomplete or temporary, local tissue ischemia (deficient blood supply) occurs. If the obstruction is complete or prolonged, local tissue death (infarction) occurs.
Venous thrombosis is usually associated with venous stasis. When blood flows slowly, thrombin and other procoagulant substances present in the blood become concentrated in local areas and initiate the clotting process. With a normal rate of blood flow, these substances are rapidly removed from the blood, primarily by Kupffer’s cells in the liver. A venous thrombus is less cohesive than an arterial thrombus, and an embolus can easily become detached and travel to other parts of the body. This embolus may be a thrombus, fat, air, amniotic fluid, tissue, or bacterial debris.
Venous thrombi cause disease by two mechanisms. First, thrombosis causes local congestion, edema, and perhaps inflammation by impairing normal outflow of venous blood (e.g., thrombophlebitis, deep vein thrombosis [DVT]). Second, embolization obstructs the blood supply when the embolus becomes lodged. The pulmonary arteries are common sites of embolization.
Pathophysiology
Atherosclerosis is the basic disease process that often leads to pathologic thrombosis. Atherosclerosis begins with accumulation of lipid-filled macrophages (i.e., foam cells) on the inner lining of arteries. Foam cells develop in response to elevated blood lipid levels and eventually become fibrous plaques (i.e., foam cells covered by smooth muscle cells and connective tissue). Advanced atherosclerotic lesions also contain hemorrhages, ulcerations, and scar tissue.
Atherosclerosis can affect any organ or tissue but often involves the arteries supplying the heart, brain, and legs. Over time, plaque lesions become larger and extend farther into the lumen of the artery. Eventually, a thrombus may develop at plaque sites and partially or completely occlude an artery. In coronary arteries, a thrombus may precipitate myocardial ischemia (angina or infarction) (see Chap. 26); in carotid or cerebral arteries, a thrombus may precipitate a stroke; in peripheral arteries, a thrombus may cause intermittent claudication (pain in the legs with exercise) or acute occlusion. Thus, serious impairment of blood flow may occur with a large atherosclerotic plaque or a relatively small plaque with superimposed vasospasm and thrombosis. Consequences and clinical manifestations of thrombi and emboli depend primarily on their location and size.
Thrombotic disorders occur much more often than bleeding disorders and are emphasized in this chapter. Bleeding disorders may result from excessive amounts of drugs that inhibit clotting.
Clinical Manifestations
Clinical manifestations of thrombosis vary depending on the size and location (arterial or venous system) of the thrombus. Symptoms are the result of decreased perfusion to an area due to the restriction or cessation of blood flow. Arterial blood clots in the cerebral, pulmonary, or cardiac system can produce a cerebrovascular accident, pulmonary embolism, or myocardial infarction, respectively. Venous blood clots may lead to DVT; classic symptoms include leg swelling and pain on palpation in the calf or thigh. However, half of all affected patients do not have any symptoms of DVT. Additional discussion of clinical manifestations is found in The Nursing Process, Assessment.
Drug Therapy
Drugs given to prevent or treat thrombosis alter some aspect of the blood coagulation process (Table 7.1; see Fig. 7.1). Anticoagulant drugs, which prevent formation of new clots and extension of already existing clots, do not dissolve clots that have already formed. Widely used in thrombotic disorders, they are more effective in preventing venous thrombosis than arterial thrombosis. Antiplatelet drugs are used to prevent arterial thrombosis. Thrombolytic drugs are used to dissolve thrombi and limit tissue damage in selected thromboembolic disorders.
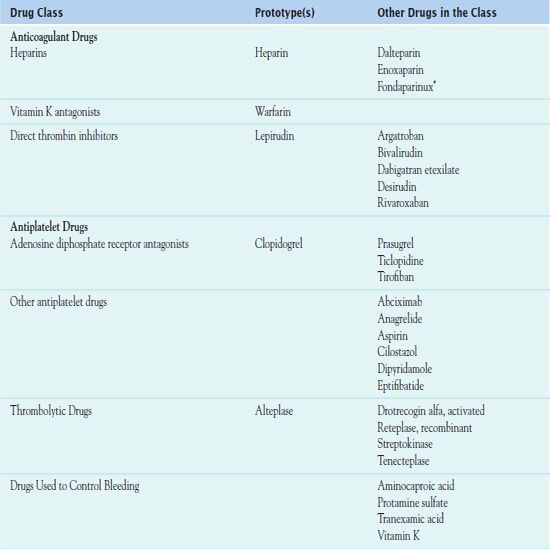
*Chemically related to low molecular weight heparins.
Anticoagulant Drugs
There are three types of anticoagulants: heparins, vitamin K antagonists, and direct thrombin inhibitors (DTIs).
Heparins
 Heparin is a pharmaceutical preparation of the natural anticoagulant produced primarily by mast cells in pericapillary connective tissue, and it is the prototype anticoagulant. Endogenous heparin is found in various body tissues, most abundantly in the liver and lungs. Exogenous heparin is obtained from bovine lung or porcine intestinal mucosa and standardized in units of biologic activity. See below for a discussion of low-molecular-weight heparins (LMWHs).
Heparin is a pharmaceutical preparation of the natural anticoagulant produced primarily by mast cells in pericapillary connective tissue, and it is the prototype anticoagulant. Endogenous heparin is found in various body tissues, most abundantly in the liver and lungs. Exogenous heparin is obtained from bovine lung or porcine intestinal mucosa and standardized in units of biologic activity. See below for a discussion of low-molecular-weight heparins (LMWHs).
Pharmacokinetics
It is necessary to give heparin intravenously or subcutaneously, because the gastrointestinal (GI) tract does not absorb the drug. After intravenous (IV) injection, it acts immediately. After subcutaneous injection, it acts within 20 to 30 minutes. Metabolism takes place in the liver and the reticuloendothelial system. Excretion, primarily in the form of inactive metabolites, occurs in the urine. Hemodialysis does not remove it.
Action
Heparin combines with antithrombin III (a natural anticoagulant in the blood) to inactivate clotting factors IX, X, XI, and XII; inhibit the conversion of prothrombin to thrombin; and prevent thrombus formation (see Fig. 7.1). After thrombosis has developed, heparin can inhibit additional coagulation by inactivating thrombin, preventing the conversion of fibrinogen to fibrin and inhibiting factor XIII (fibrin-stabilizing factor). Other effects include inhibition of factors V and VIII and platelet aggregation.
Use
Prophylactically, patients at risk for certain disorders take low doses of heparin prophylactically to prevent DVT and pulmonary embolism. These disorders include
• Major illnesses (e.g., acute myocardial infarction, heart failure, serious pulmonary infections, stroke)
• Major abdominal or thoracic surgery
• A history of thrombophlebitis or pulmonary embolism, including pregnant women
• Gynecologic surgery, especially in patients who have been taking estrogens or oral contraceptives or have other risk factors for DVT
• Restrictions such as bed rest or limited activity expected to last longer than 5 days
Therapeutically, patients receive heparin for management of acute thromboembolic disorders (e.g., DVT, thrombophlebitis, pulmonary embolism). In these conditions, the aim of therapy is to prevent further thrombus formation and embolization. Another use is in disseminated intravascular coagulation (DIC), a life-threatening condition characterized by widespread clotting, which depletes the blood of coagulation factors. The depletion of coagulation factors then produces widespread bleeding. The goal of heparin therapy in DIC is to prevent blood coagulation long enough for clotting factors to be replenished and thus be able to control hemorrhage. In addition, clinicians use heparin to prevent clotting during cardiac and vascular surgery, extra-corporeal circulation, hemodialysis, and blood transfusions, and in blood samples to be used in laboratory tests. Heparin does not cross the placental barrier and is not secreted in breast milk, making it the anticoagulant of choice for use during pregnancy and lactation.
Table 7.2 presents the dosage information for the heparins.
 TABLE 7.2
TABLE 7.2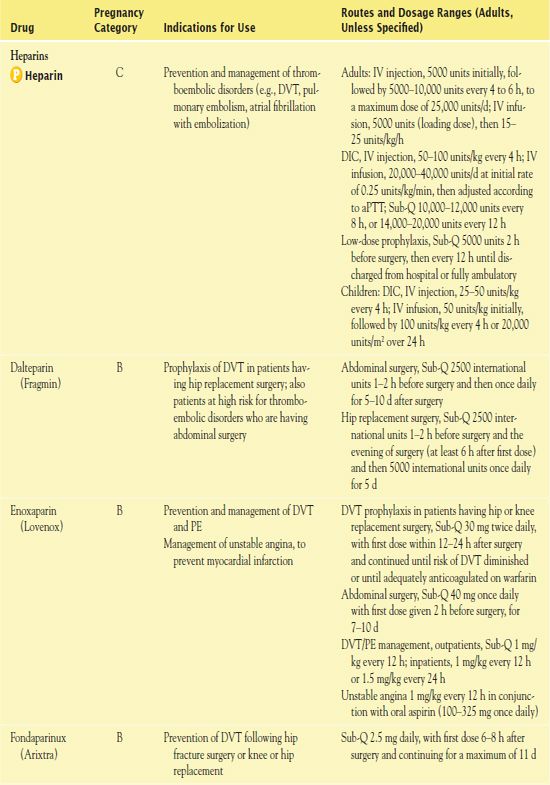
aPTT, activated partial thromboplastin time; DIC, disseminated intravascular coagulation; DVT, deep vein thrombosis; INR, international normalized ratio; PE, pulmonary embolism; PTCA, percutaneous transluminal coronary angioplasty or atherectomy; sec, seconds.
Use in Children
Little information about the use of anticoagulants in children is available. When children take heparin for systemic anti-coagulation, weight is the basis for determination of dosage (~50 units/kg). It is essential to use extreme caution to ensure that the vial concentration of heparin is correct. Fatalities in infants involving heparin overdoses have occurred. In addition, premature infants should not take heparin solutions containing benzyl alcohol as a preservative; fatal reactions have resulted.
QSEN Safety Alert 
Several major adverse events have resulted from the use of heparin, and it is classified as a high-alert drug. Nurses demonstrate consistent practice in administrating heparin by their heightened individual awareness of the risks and by advocating for systems to account for human error such as bar coding and “smart” pumps. Effective standardized practices to support safety and quality include special safeguards to reduce the risk of errors that may harm the patient.
Use in Older Adults
Older adults often have atherosclerosis and thrombotic disorders, including myocardial infarction, thrombotic stroke, and peripheral arterial insufficiency, for which they receive an anticoagulant drug. They are more likely than younger adults to experience bleeding and other complications associated with this therapy. With standard heparin, general principles for safe and effective use apply. With LMWHs, elimination may be delayed in older adults with renal impairment, and the drugs should be used cautiously.
Use in Patients With Renal Impairment
People with renal impairment may take heparin in usual dosages. However, the half-life of the drug may increase.
Use in Patients With Hepatic Impairment
Likewise, people with hepatic impairment may take heparin in usual dosages. However, the half-life of the drug may increase or decrease.
Use in Patients With Critical Illness
Heparin is often used in patients who are critically ill. However, the risk of bleeding is increased in the presence of other coexisting conditions. People who are critically ill have a high risk of DVT and pulmonary embolism, as well as a higher morbidity and mortality, including an increase in length of hospital stay, the need and duration of mechanical ventilation, and death. Effective prevention and treatment of thrombosis is necessary and typically includes LMWHs. In addition, it is essential to consider intermittent pneumatic compression devices and other measures to prevent DVT or pulmonary embolism.
Use in Patients Receiving Home Care
Patients may take standard heparin at home using the subcutaneous route, and use of LMWHs for home management of venous thrombosis has become standard practice. Daily visits by a home care nurse may be necessary if the patient or a family member is unable or unwilling to inject the medication. It is essential to take platelet counts before therapy begins and every 2 to 3 days during heparin therapy. If the platelet count falls below 100,000 platelets per microliter of blood or to less than half the baseline value, it is necessary to discontinue the heparin.
Adverse Effects
Hemorrhage is the major side effect of heparin therapy, hypersensitivity to the drug has occurred, and local irritation with subcutaneous injections of heparin can cause erythema and mild pain. Heparin-induced thrombocytopenia (HIT) (type II) is a potentially life-threatening complication of heparin administration, leading to a decrease in platelet count and detectable HIT antibodies. This condition occurs in 1% to 3% of people receiving heparin at therapeutic levels for 4 to 14 days, sometimes sooner in those who have previously received heparin. HIT is one of the most common immune-mediated adverse drug reactions. All patients exposed to any heparin at therapeutic or prophylactic doses or minute amounts in heparin flushes or on heparin-coated catheters, as well as those receiving LMWH, are at risk. If HIT occurs, it is necessary to discontinue all heparin and manage anticoagulation with a DTI such as argatroban.
Contraindications
Contraindications include GI ulcerations (e.g., peptic ulcer disease, ulcerative colitis), intracranial bleeding, dissecting aortic aneurysm, blood dyscrasias, severe kidney or liver disease, severe hypertension, polycythemia vera, and recent surgery of the eye, spinal cord, or brain. Caution is necessary in patients with hypertension, renal or hepatic disease, alcoholism, history of GI ulcerations, drainage tubes (e.g., nasogastric tubes, indwelling urinary catheters), threatened abortion, endocarditis, and any occupation with high risks of traumatic injury.
Nursing Implications
Preventing Interactions
Many medications interact with heparin, increasing or decreasing its effect (Box 7.2). Some herbs and foods increase the effects of the drug (Box 7.3). No herbs or foods that decrease the effects of heparin have been identified.
BOX 7.2  Drug Interactions: Heparin
Drug Interactions: Heparin
Drugs That Increase the Effects of Heparin
 Alteplase
Alteplase
Increases the risk of bleeding
 Antithrombin
Antithrombin
Increases pharmacologic effects
 Cephalosporins
Cephalosporins
Lead to potential coagulopathies and risk of bleeding
 Direct thrombin inhibitors
Direct thrombin inhibitors
Increase the risk of bleeding
 Drotrecogin alfa
Drotrecogin alfa
Increases the risk of bleeding
 Penicillins (parenteral)
Penicillins (parenteral)
Lead to altered platelet aggregation and increased risk of bleeding.
 Platelet inhibitors
Platelet inhibitors
Increase the risk of bleeding
 Warfarin
Warfarin
May prolong and possibly invalidate the PT; if receiving both heparin and warfarin, draw blood for the PT at least 5 hours after the last IV heparin dose
Drugs That Decrease the Effects of Heparin
 Antihistamines
Antihistamines
Decrease the anticoagulant effect
 Digoxin
Digoxin
Decreases the anticoagulant effect
 Nicotine
Nicotine
Decreases the anticoagulant effect
 Nitroglycerin (IV)
Nitroglycerin (IV)
Decreases the anticoagulant effect
 Streptokinase
Streptokinase
Leads to relative resistance to anticoagulation
 Tetracycline
Tetracycline
Decrease the anticoagulant effect
IV, Intravenous: PT prothrombin time.
BOX 7.3  Herb and Dietary Interactions: Heparin
Herb and Dietary Interactions: Heparin
Herbs and Foods That Increase the Effects of Heparin
 Chamomile, garlic, ginger, ginkgo, ginseng, high-dose vitamin E
Chamomile, garlic, ginger, ginkgo, ginseng, high-dose vitamin E
Administering the Medication
Traditional anticoagulants have two major limitations: a narrow therapeutic window of adequate anticoagulation without bleeding and a highly variable individual dose-response that requires monitoring by laboratory testing. Prescribers use the activated partial thromboplastin time (aPTT), which is sensitive to changes in blood clotting factors, except factor VII, to regulate heparin dosage. Thus, normal or control values of aPTT indicate normal blood coagulation, and therapeutic values of adequate anticoagulation indicate low levels of clotting factors and delayed blood coagulation. During heparin therapy, the aPTT should be maintained at approximately 1.5 to 2.5 times the control or baseline value. The normal control value is 25 to 35 seconds; therefore, therapeutic values of adequate anticoagulation are 45 to 70 seconds, approximately. With continuous IV infusion, blood for the aPTT may be drawn at any time; with intermittent administration, blood for the aPTT should be drawn approximately 1 hour before a dose of heparin is scheduled. It is not necessary to monitor aPTT with low-dose standard heparin given subcutaneously for prophylaxis of thromboembolism or with the LMWHs (e.g., enoxaparin).
The nurse should be aware that heparin has disadvantages: parenteral injection is necessary, and the drug has a short duration of action, which means that there is a need for frequent administration.
Assessing for Therapeutic Effects
The nurse assesses for the absence or reduction of signs and symptoms of thrombotic disorders (e.g., less edema and pain with DVT, less chest pain and respiratory difficulty with pulmonary embolism, absence of uncontrolled bleeding). It is also necessary to ensure that aPTT values are within the therapeutic range.
A Meta-Analysis of Effects of Heparin Flush and Saline Flush: Quality and Cost Implications
by GOODE, C. J., TITLER, M. RAKEL B, ET AL.
Nursing Research 1991, 40(6), 324-330
An important component of intravenous (IV) care is maintaining line patency. Until recently, heparin solutions had been used to flush catheters despite existence of evidence obtained 20 years ago demonstrating that saline flushes are as effective as heparin for maintaining patency and preventing phlebitis in peripheral devices. The use of saline prevents complications associated with use of an anticoagulant such as hemorrhage and heparin-induced thrombocytopenia. This seminal meta-analysis indicated that there is no statistical difference between the incidence of clotting and phlebitis and the duration of IV patency in saline and heparin flushes.
IMPLICATIONS FOR NURSING PRACTICE: Saline flushes are as effective as heparin solutions for maintaining patency of peripheral devices and are associated with decreased risks compared with heparin. Practice decisions must be supported by the best available evidence and not merely tradition.
Assessing for Adverse Effects
The nurse assesses the patient for signs of overt bleeding or HIT. Protamine sulfate, which is discussed in more detail later in the chapter, is an antidote for standard heparin and LMWHs. Protamine is typically given for bleeding that may not respond to merely withdrawing the heparin.
Patient Teaching
Education related to bleeding risk is essential for patients receiving heparin. The nurse reinforces instructions for safe use of the drug and related anticoagulants, reminding patients to obtain laboratory tests, and teaching how to observe for signs and symptoms of bleeding. Additional patient teaching guidelines for anticoagulants, including heparins, are outlined in Box 7.4.
BOX 7.4  Patient Teaching Guidelines for Anticoagulants
Patient Teaching Guidelines for Anticoagulants
General Considerations
 Anticoagulant drugs are given to people who have had, or who are at risk of having, a heart attack, stroke, or other problems from blood clots. For home management of deep vein thrombosis, which usually occurs in the legs, you are likely to be given heparin injections for a few days, followed by warfarin for long-term therapy. These medications help prevent the blood clot from getting larger, traveling to your lungs, or recurring later.
Anticoagulant drugs are given to people who have had, or who are at risk of having, a heart attack, stroke, or other problems from blood clots. For home management of deep vein thrombosis, which usually occurs in the legs, you are likely to be given heparin injections for a few days, followed by warfarin for long-term therapy. These medications help prevent the blood clot from getting larger, traveling to your lungs, or recurring later.
 All anticoagulants can increase the risk of bleeding, so you need to take safety precautions to prevent injury.
All anticoagulants can increase the risk of bleeding, so you need to take safety precautions to prevent injury.
 To help prevent blood clots from forming and decreasing blood flow through your arteries, you need to reduce risk factors that contribute to cardiovascular disease. This can be done by a low-fat, low-cholesterol diet (and medication if needed) to lower total cholesterol to below 200 mg/dL and low-density lipoprotein cholesterol to below 130 mg/dL; weight reduction if overweight; control of blood pressure if hypertensive; avoidance of smoking; stress-reduction techniques; and regular exercise.
To help prevent blood clots from forming and decreasing blood flow through your arteries, you need to reduce risk factors that contribute to cardiovascular disease. This can be done by a low-fat, low-cholesterol diet (and medication if needed) to lower total cholesterol to below 200 mg/dL and low-density lipoprotein cholesterol to below 130 mg/dL; weight reduction if overweight; control of blood pressure if hypertensive; avoidance of smoking; stress-reduction techniques; and regular exercise.
 To help maintain a steady level of anticoagulation with warfarin, do not change your intake of foods that are high in vitamin K, which decreases the effects of warfarin. These foods include broccoli, brussels sprouts, cabbage, cauliflower, chives, collard greens, kale, lettuce, mustard greens, peppers, spinach, tomatoes, turnips, and watercress.
To help maintain a steady level of anticoagulation with warfarin, do not change your intake of foods that are high in vitamin K, which decreases the effects of warfarin. These foods include broccoli, brussels sprouts, cabbage, cauliflower, chives, collard greens, kale, lettuce, mustard greens, peppers, spinach, tomatoes, turnips, and watercress.
 To help prevent blood clots from forming in your leg veins, avoid or minimize situations that slow blood circulation, such as wearing tight clothing, crossing the legs at the knees, prolonged sitting or standing, and bed rest. For example, on automobile trips, stop and walk around every 1 to 2 hours; on long plane trips, exercise your feet and legs at your seat and walk around when you can.
To help prevent blood clots from forming in your leg veins, avoid or minimize situations that slow blood circulation, such as wearing tight clothing, crossing the legs at the knees, prolonged sitting or standing, and bed rest. For example, on automobile trips, stop and walk around every 1 to 2 hours; on long plane trips, exercise your feet and legs at your seat and walk around when you can.
 Following instructions regarding these medications is extremely important. Too little medication increases your risk of problems from blood clot formation; too much medication can cause bleeding.
Following instructions regarding these medications is extremely important. Too little medication increases your risk of problems from blood clot formation; too much medication can cause bleeding.
 While taking any of these medications, you need regular medical supervision and periodic blood tests. The blood tests can help your health care provider regulate drug dosage and maintain your safety.
While taking any of these medications, you need regular medical supervision and periodic blood tests. The blood tests can help your health care provider regulate drug dosage and maintain your safety.
 Notify your health care provider if you suddenly stop tobacco smoking, because this may result in a reduced clearance of warfarin. A dosage change may be necessary.
Notify your health care provider if you suddenly stop tobacco smoking, because this may result in a reduced clearance of warfarin. A dosage change may be necessary.
 With enoxaparin, you need an injection, usually every 12 hours. You or someone close to you may be instructed in injecting the medication, or a visiting nurse may do the injections, if necessary.
With enoxaparin, you need an injection, usually every 12 hours. You or someone close to you may be instructed in injecting the medication, or a visiting nurse may do the injections, if necessary.
 You need to take the drugs as directed. Avoid taking other drugs without the health care provider’s knowledge and consent, inform any health care provider (including dentists) that you are taking an anticoagulant drug before any invasive diagnostic tests or treatments are begun, and keep all appointments for continuing care.
You need to take the drugs as directed. Avoid taking other drugs without the health care provider’s knowledge and consent, inform any health care provider (including dentists) that you are taking an anticoagulant drug before any invasive diagnostic tests or treatments are begun, and keep all appointments for continuing care.
 With warfarin therapy, you need to avoid walking barefoot; avoid contact sports; use an electric razor; avoid injections when possible; and carry an identification card, necklace, or bracelet (e.g., MedicAlert) stating the name of the drug and the health care provider’s name and telephone number.
With warfarin therapy, you need to avoid walking barefoot; avoid contact sports; use an electric razor; avoid injections when possible; and carry an identification card, necklace, or bracelet (e.g., MedicAlert) stating the name of the drug and the health care provider’s name and telephone number.
 A routine blood test is necessary to ensure that your warfarin dose is appropriate. The results of this test determine your daily dose of warfarin. Once the warfarin dose stabilizes, the blood tests are done less often (e.g., every 2 weeks).
A routine blood test is necessary to ensure that your warfarin dose is appropriate. The results of this test determine your daily dose of warfarin. Once the warfarin dose stabilizes, the blood tests are done less often (e.g., every 2 weeks).
 Report any sign of bleeding (e.g., excessive bruising of the skin, blood in urine or stool). If superficial bleeding occurs, apply direct pressure to the site for 3 to 5 minutes or longer if necessary.
Report any sign of bleeding (e.g., excessive bruising of the skin, blood in urine or stool). If superficial bleeding occurs, apply direct pressure to the site for 3 to 5 minutes or longer if necessary.
Self-Administration
 With enoxaparin, wash hands and cleanse skin to prevent infection; inject deep under the skin, around the navel, upper thigh, or buttocks; and change the injection site daily. If excessive bruising occurs at the injection site, rubbing an ice cube over an area before the injection may be helpful.
With enoxaparin, wash hands and cleanse skin to prevent infection; inject deep under the skin, around the navel, upper thigh, or buttocks; and change the injection site daily. If excessive bruising occurs at the injection site, rubbing an ice cube over an area before the injection may be helpful.
 With warfarin as with all medications, take as prescribed. Because the prescriber may set a dosing schedule that could vary from 1 day to the next, do not rely on memory but keep a written record of the date and the amount of medication taken.
With warfarin as with all medications, take as prescribed. Because the prescriber may set a dosing schedule that could vary from 1 day to the next, do not rely on memory but keep a written record of the date and the amount of medication taken.
Other Drugs in the Class
Standard heparin is a mixture of high and low molecular weight fractions, but most anticoagulant activity is attributed to the low molecular weight portion. LMWHs contain the low molecular weight fraction and are as effective as IV heparin in treating thrombotic disorders. Indications for their use include prevention or management of thromboembolic complications associated with surgery or ischemic complications of unstable angina and myocardial infarction. Currently available LMWHs (dalteparin, enoxaparin) differ from standard heparin and each other and are not interchangeable.
LMWHs are given subcutaneously and do not require close monitoring of blood coagulation. These characteristics allow outpatient anticoagulant therapy, an increasing standard. The drugs are also associated with less thrombocytopenia than standard heparin. However, monitoring of platelet counts during therapy is necessary.
Clinical Application 7-1
 To regulate the amount of heparin Mr. Oliver receives, his aPTT is measured. What is the therapeutic value for aPTT?
To regulate the amount of heparin Mr. Oliver receives, his aPTT is measured. What is the therapeutic value for aPTT?
Vitamin K Antagonists
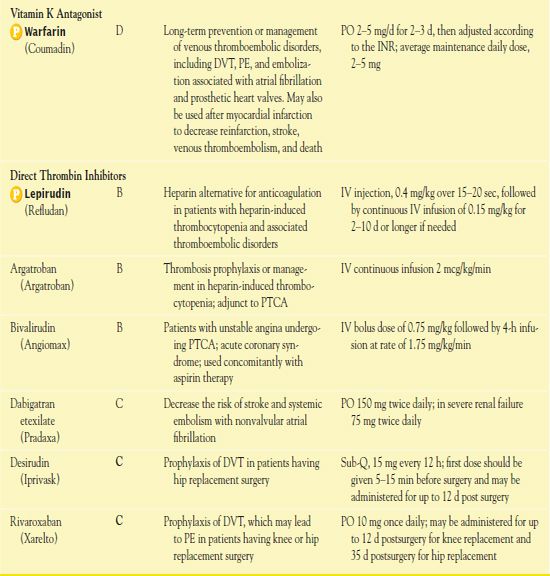
 Warfarin (Coumadin) is the most commonly used oral anticoagulant and is the prototype vitamin K antagonist. Table 7.2 presents dosage information for warfarin.
Warfarin (Coumadin) is the most commonly used oral anticoagulant and is the prototype vitamin K antagonist. Table 7.2 presents dosage information for warfarin.
Pharmacokinetics
Warfarin is well absorbed after oral administration. Administration with food may delay the rate but not the extent of absorption. The drug is highly bound to plasma proteins (98%), mainly albumin. Metabolism takes place in the liver. Excretion, primarily as inactive metabolites, occurs in the kidneys. Renal impairment does not affect drug metabolism but may decrease excretion of the drug.
Action
Warfarin acts in the liver to prevent synthesis of vitamin K– dependent clotting factors (i.e., factors II, VII, IX, and X). Similar to vitamin K in structure, warfarin therefore acts as a competitive antagonist to hepatic use of vitamin K. Conversely, vitamin K serves as the antidote for warfarin. Warfarin has no effect on circulating clotting factors or on platelet function, so the anticoagulant effects do not occur for 3 to 5 days after warfarin is started because clotting factors already in the blood follow their normal pathway of elimination.
Use
Warfarin is most useful in long-term prevention or management of venous thromboembolic disorders, including DVT, pulmonary embolism, and embolization associated with atrial fibrillation and prosthetic heart valves. In addition, warfarin therapy after myocardial infarction may decrease reinfarction, stroke, venous thromboembolism, and death. The smaller doses used now are equally effective as ones used formerly, with similar antithrombotic effects and decreased risks of bleeding.
Use in Children
After cardiac surgery, children receive warfarin to prevent thromboembolism, but there are no established doses and guidelines for safe, effective use. Accurate drug administration, close monitoring of blood coagulation tests, safety measures to prevent trauma and bleeding, avoiding interacting drugs, and informing others in the child’s environment (e.g., teachers, babysitters, health care providers) are necessary.
Use in Older Adults
Warfarin metabolism may be altered in older adults. As patient age increases, a lower dose of warfarin is usually required to produce a therapeutic effect.
Use in Patients With Hepatic Impairment
Warfarin is more likely to cause bleeding in patients with hepatic disease because of decreased synthesis of vitamin K and decreased plasma proteins. In addition, only the liver eliminates warfarin; thus, it may accumulate in people with hepatic impairment, and dosage adjustment may be necessary.
Use in Patients With Critical Illness
Because the anticoagulant and antithrombotic effects of warfarin take several days to occur, patients who are critically ill require concurrent treatment with other anticoagulants, such as heparin or LMWHs. Heparin is usually continued until the international normalized ratio (INR) is the therapeutic range.
Use in Patients Receiving Home Care
For prevention of DVT, warfarin is usually self-administered at home, with periodic office or clinic visits for blood tests and other follow-up care. For home management of DVT, warfarin may be self-administered, but a nurse usually visits, performs a fingerstick INR, and notifies the prescriber, who then prescribes the appropriate dose of warfarin. Precautions to decrease risks of bleeding are necessary. However, the risk of bleeding has decreased in recent years because lower doses of warfarin are now used. In addition, medical conditions other than anticoagulation may cause bleeding during warfarin therapy.
Adverse Effects
The primary adverse effect associated with warfarin therapy is hemorrhage. Additionally, nausea, vomiting, abdominal pain, alopecia, urticaria, dizziness, and joint or muscle pain may occur.
Contraindications
Contraindications to warfarin include GI ulcerations, blood disorders associated with bleeding, severe kidney or liver disease, severe hypertension, and recent surgery of the eye, spinal cord, or brain. Caution is warranted in patients with mild hypertension, renal or hepatic disease, alcoholism, history of GI ulcerations, drainage tubes (e.g., nasogastric tubes, indwelling urinary catheters), and occupations with high risks of traumatic injury. Warfarin, a pregnancy category X medication, is contraindicated during pregnancy because it crosses the placenta and may produce fatal fetal hemorrhage. The Food and Drug Administration (FDA) has issued a BLACK BOX WARNING ♦ for warfarin due to its risk of causing major or fatal bleeding.
Nursing Implications
Preventing Interactions
Many medications and herbs interact with warfarin, increasing or decreasing its effect (Boxes 7.5 and 7.6).
BOX 7.5  Drug Interactions: Warfarin
Drug Interactions: Warfarin



