CHAPTER 3 In 1962, Congress passed the Harris-Kefauver Amendments to the Food, Drug and Cosmetic Act. This law was created in response to the thalidomide tragedy that occurred in Europe in the early 1960s. Thalidomide is a sedative now known to cause birth defects and fetal death. Because the drug was used widely by pregnant women, thousands of infants were born with phocomelia, a rare birth defect characterized by the gross malformation or complete absence of arms or legs. This tragedy was especially poignant in that it resulted from nonessential drug use: The women who took thalidomide could have done very well without it. Thalidomide was not a problem in the United States because the drug never received approval by the FDA (see Chapter 107, Box 107–1). In 1970, Congress passed the Controlled Substances Act (Title II of the Comprehensive Drug Abuse Prevention and Control Act). This legislation set rules for the manufacture and distribution of drugs considered to have the potential for abuse. One provision of the law defines five categories of controlled substances, referred to as Schedules I, II, III, IV, and V. Drugs in Schedule I have no accepted medical use in the United States and are deemed to have a high potential for abuse. Examples include heroin, mescaline, and lysergic acid diethylamide (LSD). Drugs in Schedules II through V have accepted medical applications but also have the potential for abuse. The abuse potential of these agents becomes progressively less as we proceed from Schedule II to Schedule V. The Controlled Substances Act is discussed further in Chapter 37 (Drug Abuse: Basic Considerations). • The fast-track system created for AIDS drugs and cancer drugs now includes drugs for other serious and life-threatening illnesses. • Manufacturers who plan to stop making a drug must inform patients at least 6 months in advance, thereby giving them time to find another source. • A clinical trial database will be established for drugs directed at serious or life-threatening illnesses. These data will allow clinicians and patients to make informed decisions about using experimental drugs. • Drug companies can now give prescribers journal articles and certain other information regarding “off-label” uses of drugs. (An “off-label” use is a use that has not been evaluated by the FDA.) Prior to the new act, clinicians were allowed to prescribe a drug for an off-label use, but the manufacturer was not allowed to promote the drug for that use—even if promotion was limited to providing potentially helpful information, including reprints of journal articles. In return for being allowed to give prescribers information regarding off-label uses, manufacturers must promise to do research to support the claims made in the articles. The testing of new drugs has two principal steps: preclinical testing and clinical testing. Preclinical tests are performed in animals. Clinical tests are done in humans. The steps in drug development are outlined in Table 3–1. TABLE 3–1 The hidden dangers in new drugs are illustrated by the data in Table 3–2. This table presents information on 11 drugs that were withdrawn from the U.S. market soon after receiving FDA approval. In all cases, the reason for withdrawal was a serious adverse effect that went undetected in clinical trials. Admittedly, only a few hidden adverse effects are as severe as the ones in the table. Hence, most do not necessitate drug withdrawal. Nonetheless, the drugs in the table should serve as a strong warning about the unknown dangers that a new drug may harbor. TABLE 3–2 Some New Drugs That Were Withdrawn from the U.S. Market for Safety Reasons
Drug regulation, development, names, and information
Landmark drug legislation
New drug development
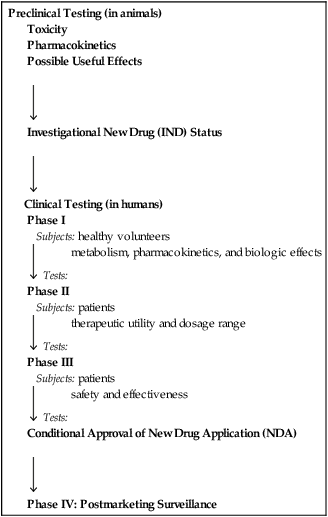
Stages of new drug development

Preclinical Testing (in animals)
Toxicity
Pharmacokinetics
Possible Useful Effects
Investigational New Drug (IND) Status
Clinical Testing (in humans)
Phase I
Subjects: healthy volunteers
 Tests: metabolism, pharmacokinetics, and biologic effects
Tests: metabolism, pharmacokinetics, and biologic effects
Phase II
Subjects: patients
 Tests: therapeutic utility and dosage range
Tests: therapeutic utility and dosage range
Phase III
Subjects: patients
 Tests: safety and effectiveness
Tests: safety and effectiveness
Conditional Approval of New Drug Application (NDA)
Phase IV: Postmarketing Surveillance
Limitations of the testing procedure
Failure to detect all adverse effects

Drug
Indication
Year Introduced/Year Withdrawn
Months on the Market
Reason for Withdrawal
Rotigotine* [Neupro]
Parkinson’s disease
2007/2008
10
Patch formulation delivered erratic dosages
Natalizumab† [Tysabri]
Multiple sclerosis
2004/2005
3
Progressive multifocal leukoencephalopathy
Rapacuronium [Raplon]
Neuromuscular blockade
1999/2001
19
Bronchospasm, unexplained fatalities
Alosetron† [Lotronex]
Irritable bowel syndrome
2000/2000
9
Ischemic colitis, severe constipation; deaths have occurred
Troglitazone [Rezulin]
Type 2 diabetes
1999/2000
12
Fatal liver failure
Grepafloxacin [Raxar]
Infection
1997/1999
19
Severe cardiovascular events, including seven deaths
Bromfenac [Duract]
Acute pain
1997/1998
11
Severe hepatic failure
Mibefradil [Posicor]
Hypertension, angina pectoris
1997/1998
11
Inhibits drug metabolism, causing toxic accumulation of many drugs
Dexfenfluramine [Redux]
Obesity
1996/1997
16
Valvular heart disease
Flosequinan [Manoplax]
Heart failure
1992/1993
4
Increased hospitalization; decreased survival
Temafloxacin [Omniflox]
Infection
1992/1992
4
Hypoglycemia; hemolytic anemia, often associated with renal failure, hepatotoxicity, and coagulopathy ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


