Dilution
May be given undiluted. Available as 2 mcg/mL solution in 2-mL vials.
Storage:
Store unopened vials at 25° C (77° F); range: 15° to 30° C (59° to 86° F). Protect from light. Discard unused portion.
Compatibility
Specific information not available. Consider specific use; consult pharmacist.
Rate of administration
Administer as a bolus dose at the end of dialysis.
Actions
A synthetic vitamin D analog. Metabolizes to a naturally occurring, biologically active form of vitamin D2 that regulates blood calcium at levels required for essential body functions (i.e., intestinal absorption of dietary calcium, tubular reabsorption of calcium by the kidney and, in conjunction with the parathyroid hormone [PTH], the mobilization of calcium from the skeleton). Acts directly on bone cells (osteoblasts) to stimulate skeletal growth and on the parathyroid glands to suppress PTH synthesis and secretion. In uremic patients, deficient production of biologically active vitamin D metabolites leads to secondary hyperparathyroidism, which contributes to the development of metabolic bone disease. Doxercalciferol is activated in the liver. Peak blood levels are reached in 2.1 to 13.9 hours. Mean half-life range is 32 to 96 hours.
Indications and uses
Reduction of elevated iPTH levels in the management of secondary hyperparathyroidism in patients undergoing chronic renal dialysis.
Contraindications
Evidence of vitamin D toxicity, hypercalcemia, hyperphosphatemia, or known hypersensitivity to any ingredient in this product; see Precautions.
Precautions
Overdose of any form of vitamin D, including doxercalciferol, is dangerous. Progressive hypercalcemia due to overdose of vitamin D and its metabolites may require emergency attention. Acute hypercalcemia may exacerbate tendencies for cardiac arrhythmias and seizures and may potentiate the action of digoxin drugs. Chronic administration may place patient at risk of hypercalcemia, elevated Ca × P product, and generalized vascular and other soft-tissue calcification. If clinically significant hypercalcemia develops, dose should be reduced or held. Do not allow the Ca × P product to exceed 55 mg2/dL2. See Side Effects and Antidote. ■ To avoid possible additive effects and hypercalcemia, phosphate or vitamin D-related compounds should not be taken concomitantly with doxercalciferol. ■ Oversuppression of iPTH levels may lead to adynamic bone syndrome. ■ Hyperphosphatemia can exacerbate hyperparathyroidism. ■ Use caution in patients with impaired hepatic function. More frequent monitoring of iPTH, calcium, and phosphorus levels is recommended. ■ Patients with higher pretreatment serum levels of calcium (more than 10.5 mg/dL) or phosphorus (more than 6.9 mg/dL) may be more likely to experience hypercalcemia or hyperphosphatemia; see Contraindications.
Monitor:
During initiation of therapy, obtain baseline serum iPTH, calcium, and phosphorus levels, and determine levels weekly during the early phase of treatment (i.e., first 12 weeks). For dialysis patients, serum or plasma iPTH and serum calcium, phosphorus, and alkaline phosphatase should be determined periodically. See Dose Adjustments. ■ Calculate Ca × P (should be less than 55 mg2/dL2). ■ Monitor serum calcium levels weekly after all dose changes and during subsequent dose titration. ■ Monitor for signs and symptoms of hypercalcemia. See Side Effects. Radiographic evaluation of suspect anatomical regions may be useful in the early detection of generalized vascular or other soft-tissue calcification. ■ Oral calcium-based or other non–aluminum-containing phosphate binders and a low phosphate diet are indicated to control serum phosphorus levels in dialysis patients. Hyperphosphatemia can lessen the effectiveness of doxercalciferol in reducing blood PTH levels. After initiating doxercalciferol therapy, the dose of calcium-containing phosphate binders should be decreased to correct persistent mild hypercalcemia (10.6 to 11.2 mg/dL for 3 consecutive determinations), or increased to correct persistent mild hyperphosphatemia (7 to 8 mg/dL for 3 consecutive determinations). ■ Persistent or markedly elevated serum calcium levels may be corrected by dialysis against a reduced calcium or calcium-free dialysate. ■ See Precautions and Drug/Lab Interactions.
Patient education:
Report symptoms of hypercalcemia promptly. Dose adjustment or treatment may be required. Strict adherence to dietary supplementation of calcium and restriction of phosphorus is required to ensure optimal effectiveness of therapy. Phosphate-binding compounds (e.g., calcium acetate [Phos-lo]) may be needed to control serum phosphorus levels in patients with CRF, but excessive use of aluminum-containing products (e.g., aluminum hydroxide gel [Alternagel]) should be avoided. ■ Review all non-prescription drugs with physician.
Maternal/child:
Category B: safety for use in pregnancy not established; use only if clearly needed. ■ Discontinue breast-feeding. ■ Safety and effectiveness for use in pediatric patients not established.
Elderly:
Drug/lab interactions
Specific interaction studies have not been performed. ■ Digoxin toxicity is potentiated by hypercalcemia. Use caution when doxercalciferol is prescribed concomitantly with digoxin compounds. ■ Phosphate or vitamin D–related compounds should not be taken concomitantly with doxercalciferol. ■ May reduce serum total alkaline phosphatase levels. ■ Magnesium-containing antacids may cause hypermagnesemia; concomitant use is not recommended. ■ Concomitant use with cytochrome P450 enzyme inducers (e.g., glutethimide, phenobarbital [Luminal], phenytoin [Dilantin], rifampin [Rifadin]) may affect hydroxylation of doxercalciferol and require dose adjustments. ■ Concomitant use with cytochrome P450 enzyme inhibitors (e.g., erythromycin, ketoconazole [Nizoral]) may inhibit metabolism of the active form of vitamin D, decreasing effectiveness.
Side effects
Dose-limiting side effects are hypercalcemia, hyperphosphatemia, and oversuppression of iPTH (less than 150 pg/mL). Overdose or chronic administration may lead to hypercalcemia. Signs and symptoms of vitamin D intoxication associated with hypercalcemia include: Early: anorexia, bone pain, constipation, dry mouth, headache, metallic taste, muscle pain, nausea, somnolence, vomiting, and weakness. Late: albuminuria, anorexia, apathy, arrested growth, cardiac arrhythmias, conjunctivitis (calcific), death, decreased libido, dehydration, ectopic calcification, elevated AST and ALT, elevated BUN, hypercholesterolemia, hypertension, hyperthermia, nocturia, overt psychosis (rare), pancreatitis, photophobia, polydipsia, polyuria, pruritus, rhinorrhea, sensory disturbances, somnolence, urinary tract infections, and weight loss.
Overdose:
Hypercalcemia, hypercalciuria, hyperphosphatemia, and oversuppression of PTH secretion leading in certain cases to adynamic bone disease. High intake of calcium and phosphate concomitant with doxercalciferol may lead to similar abnormalities. High levels of calcium in the dialysate bath may contribute to hypercalcemia.
Antidote
Notify physician of any side effects. Treatment of patients with clinically significant hypercalcemia (more than 1 mg/dL above the upper limit of normal range) consists of immediate dose reduction or interruption of the therapy and includes a low-calcium diet, withdrawal of calcium supplements, patient mobilization, attention to fluid and electrolyte imbalances, assessment of electrocardiographic abnormalities (critical in patients receiving digoxin), forced diuresis, and hemodialysis or peritoneal dialysis against a calcium-free dialysate, as warranted. Monitor serum calcium levels frequently until calcium levels return to within normal limits. Not removed from blood during hemodialysis. When serum calcium levels return to within normal limits (usually 2 to 7 days), therapy may be restarted at a dose that is at least 1 mcg lower than prior therapy.
Doxorubicin hydrochloride  ■ doxorubicin hydrochloride liposomal injection
■ doxorubicin hydrochloride liposomal injection 
(dox-oh-ROO-bih-sin hy-droh-KLOR-eyed)
(dox-oh-ROO-bih-sin hy-droh-KLOR-eyed LIP-oh-sohm-ul)
ADR, Adriamycin ■ Doxil
Antineoplastic (anthracycline antibiotic)
pH 3.8 to 6.5 ■ pH 6.5
Usual dose
Assessment required before dosing; see Precautions and Monitor.
Conventional doxorubicin
60 to 75 mg/M2 once every 21 days as a single agent. When used in combination with other agents, the most common dose of doxorubicin is 40 to 75 mg/M2 every 21 to 28 days.
Breast cancer with lymph node involvement after resection:
Doxorubicin 60 mg/M2 in combination with cyclophosphamide 600 mg/M2 given IV sequentially on Day 1 of each 21-day treatment cycle. Four cycles have been administered.
Adjuvant treatment of operable node-positive breast cancer:
Treatment protocol includes doxorubicin, cyclophosphamide, and docetaxel. Administer doxorubicin 50 mg/M2 and cyclophosphamide 500 mg/M2. One hour later, give docetaxel 75 mg/M2. Repeat every 3 weeks for 6 cycles. See docetaxel and cyclophosphamide monographs.
Doxil (liposomal doxorubicin)
Do not substitute Doxil for conventional doxorubicin.
AIDS-related Kaposi’s sarcoma:
20 mg/M2 as an IV infusion over 60 minutes every 21 days until disease progression or unacceptable toxicity.
Ovarian cancer:
50 mg/M2 as an IV infusion over 60 minutes every 28 days until disease progression or unacceptable toxicity.
Multiple myeloma:
Given in combination with bortezomib (Velcade).
Bortezomib:
Administer 1.3 mg/M2 as an IV bolus on Days 1, 4, 8, and 11 of each 21-day cycle; see bortezomib monograph.
Doxil:
Administer 30 mg/M2 as an IV infusion over 60 minutes on Day 4 of each 21-day cycle following bortezomib. Continue regimen for 8 cycles or until disease progression or the occurrence of unacceptable toxicity.
Breast cancer (unlabeled):
50 mg/M2 as an IV infusion over 60 minutes every 4 weeks. Other combination protocols are in use.
Pediatric dose
Conventional doxorubicin:
In combination with other chemotherapeutic agents as first-line treatment, 30 to 60 mg/M2 every 21 to 42 days. See Maternal/Child.
Doxil:
Safety for use in pediatric patients not established.
Dose adjustments
All doxorubicins:
Elevated serum bilirubin:
Give 50% of above doses for serum bilirubin from 1.2 to 3 mg/mL and 25% for serum bilirubin above 3 mg/mL. Discontinue therapy for serum bilirubin greater than 5 mg/dL (doxorubicin prescribing information). ■ Reduce dose in patients with impaired hepatic function. ■ See Precautions.
Conventional doxorubicin:
Consider lower-end doses or longer intervals between cycles for heavily pretreated patients, elderly patients, or obese patients.
Breast cancer with lymph node involvement after resection:
Reduce dose to 75% of the starting dose for neutropenic fever/infection. If necessary, delay the next cycle of treatment until the ANC is 1,000 cells/mm3 or more and the platelet count is 100,000 cells/mm3 or more and nonhematologic toxicities have resolved.
Doxil:
Dose adjustments are required in hematologic toxicity (see the following chart) and in patients with stomatitis or hand-foot syndrome (HFS) (see product literature for guidelines). Adjust or delay a dose as described in the product literature at the first sign of a Grade 2 or higher adverse event. Do not increase Doxil dose after a dose reduction for toxicity.
| Doxil Dosing Based on Hematologic Toxicity (Neutropenia or Thrombocytopenia) | |
| Grade | Modification |
| 1 | Resume treatment with no dose reduction. |
| 2 | Delay until ANC ≥1,500 and platelets ≥75,000; resume treatment at previous dose. |
| 3 | Delay until ANC ≥1,500 and platelets ≥75,000; resume treatment at previous dose. |
| 4 | Delay until ANC ≥1,500 and platelets ≥75,000; resume at 25% dose reduction or continue previous dose with prophylactic granulocyte growth factor. |

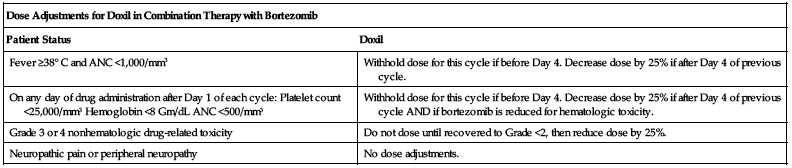
Dose adjustments for Doxil in combination therapy with bortezomib for treatment of multiple myeloma are listed in the following chart. See bortezomib monograph for bortezomib dose adjustments.
| Dose Adjustments for Doxil in Combination Therapy with Bortezomib | |
| Patient Status | Doxil |
| Fever ≥38° C and ANC <1,000/mm3 | Withhold dose for this cycle if before Day 4. Decrease dose by 25% if after Day 4 of previous cycle. |
| On any day of drug administration after Day 1 of each cycle: Platelet count <25,000/mm3 Hemoglobin <8 Gm/dL ANC <500/mm3 | Withhold dose for this cycle if before Day 4. Decrease dose by 25% if after Day 4 of previous cycle AND if bortezomib is reduced for hematologic toxicity. |
| Grade 3 or 4 nonhematologic drug-related toxicity | Do not dose until recovered to Grade <2, then reduce dose by 25%. |
| Neuropathic pain or peripheral neuropathy | No dose adjustments. |
Dilution
Specific techniques required; see precautions.
Conventional doxorubicin:
Each 10 mg must be diluted with 5 mL of NS to obtain a final concentration of 2 mg/mL. Do not use bacteriostatic diluent. Shake gently to dissolve completely. Also available in preservative-free solutions. May be further diluted in 50 mL or more D5W or NS and given as a continuous infusion through a central venous line.
Doxil:
Doses up to 90 mg must be diluted in 250 mL D5W. Not a clear solution, but a translucent red liposomal dispersion. Do not use filters. Doses over 90 mg should be diluted in 500 mL D5W. See Compatibility.
Filters:
Conventional doxorubicin:
Data not available from manufacturer; however, one source indicates no evidence of drug loss when administered through a 0.2-micron in-line nylon filter, and another source indicates no significant drug loss using various types of 0.2-micron filters.
Doxil:
Do not use filters during preparation or administration.
Storage:
Conventional doxorubicin:
Retain vials in carton until time of use. Refrigerate unopened vials containing solution. Refrigeration can result in the formation of a gelled product. If this occurs, place vial at RT for 2 to 4 hours to return the product to a slightly viscous mobile solution. Vials containing lyophilized powder may be stored at CRT. All forms should be protected from light.
Doxil:
Refrigerate unopened vials at 2° to 8° C (36° to 46° F). Do not freeze. Refrigerate diluted solution and use within 24 hours.
Compatibility (underline indicates conflicting compatibility information)
Consider any drug NOT listed as compatible to be INCOMPATIBLE until consulting a pharmacist; specific conditions may apply.
Conventional doxorubicin
Manufacturer lists fluorouracil and heparin as incompatible and states mixing with other drugs is not recommended unless specific compatibility data available. Avoid contact with alkaline solutions; can lead to hydrolysis of doxorubicin.
One source suggests the following compatibilities:
Additive:
Bleomycin (Blenoxane), cyclophosphamide (Cytoxan), dacarbazine (DTIC), ondansetron (Zofran), paclitaxel (Taxol), vinblastine, vincristine.
Y-site:
Amifostine (Ethyol), anidulafungin (Eraxis), aztreonam (Azactam), bleomycin (Blenoxane), caspofungin (Cancidas), cisplatin, cladribine (Leustatin), cyclophosphamide (Cytoxan), doripenem (Doribax), droperidol (Inapsine), etoposide phosphate (Etopophos), filgrastim (Neupogen), fludarabine (Fludara), fluorouracil (5-FU), gemcitabine (Gemzar), granisetron (Kytril), leucovorin calcium, linezolid (Zyvox), melphalan (Alkeran), methotrexate, metoclopramide (Reglan), mitomycin (Mutamycin), ondansetron (Zofran), oxaliplatin (Eloxatin), paclitaxel (Taxol), sargramostim (Leukine), sodium bicarbonate, teniposide (Vumon), thiotepa, topotecan (Hycamtin), vinblastine, vincristine, vinorelbine (Navelbine).
Doxil
Specific information not available. Manufacturer states, “Do not mix Doxil with other drugs. Do not use any other diluent (use D5W only). Do not use any bacteriostatic agents (e.g., benzyl alcohol).”
One source suggests the following compatibilities:
Y-site:
Not recommended by manufacturer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


