Computed Tomography
CT, first introduced in 1972 by Hounsfield,
2 is based on the use of conventional ionized radiation that is attenuated as it passes through the skull, cerebrospinal fluid (CSF), cerebral gray and white matter, and blood vessels. More than thirty thousand 2- to 4-mm x-ray beams are directed successively at several horizontal levels in any plane.
3 Detectors measure the degree of attenuation of the exiting radiation. Computers integrate this information and construct the images as cross-section images. The thickness of a cross-section, which is called a
cut or
slice, can vary from 1.5 to 10 mm although most cuts are no more than 5 mm to allow for greater
precision in identifying small abnormal areas. Each cut is subdivided into a grid of tiny cubes or volume elements called
voxels. The degree of attenuation within each voxel is measured as a numeric value of tissue density and is converted to gray scale values with lower numbers coded as black (i.e., air, CSF) and higher numbers coded as white (i.e., bone). The differing densities of bone, CSF, blood, and gray and white matter are distinguishable in the resulting picture. Films of the various cuts are then arranged sequentially for review.
Clinical Applications
The American College of Radiology and American Society of Neuroradiology
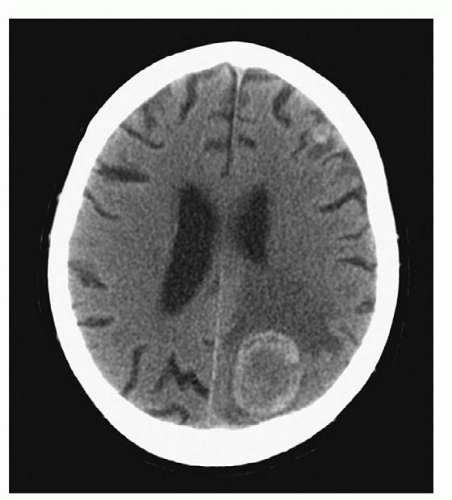
4 have published guidelines for use of CTs in clinical practice. The more recent generation of CT scanners provide extraordinary clarity of the brain, orbits, and spine. Detailed visualization of sulci, caudate and lenticular nuclei, the internal capsule, thalami, optic nerves, the brainstem, the cerebellum, and the spinal cord is provided. One can visualize hemorrhage, cerebral edema, abscess, and tumor as well as the precise size and position of the ventricles and midline structures. A CT can be ordered with or without contrast. A CT without contrast is ordered to rule out bleeding or a hematoma when a cerebral aneurysm is suspected, and to screen for hemorrhage in candidates for thrombolytic therapy with a diagnosis of ischemic stroke. A CT with contrast is ordered when more precise detail is desired. Serial CT scans can be used to follow the resolution of cerebral hemorrhage or edema (
Fig. 6-3).
Recent advances in CT technology of spiral and helical CT scans have greatly increased the speed of the scanning procedure and have made possible the visualization of cerebral vasculature (i.e., CTA).
3 The newest addition to CT technology is a portable CT scanner. The portability of this equipment allows the scanner to be brought to the emergency room or patient’s bedside (e.g., intensive care unit [ICU]; other units) for scanning and immediate availability of quality images for review. NeuroLogica Corporation (Danvers, MA) offers light weight, high speed, and portable scanners called CereTom. The portable scanner is especially useful in the ICU environment in that the patient does not need to be disconnected from monitoring devices such as the intracranial pressure (ICP) monitor or ventilator.
5 Transporting a patient from the ICU to a radiology site for scanning has always placed the patient at high risk for unexpected events such as desaturation, hypotension, hemodynamic instability, dislodgement of endotracheal tube or other lines, aspiration, and cardiac arrest. Avoiding such transport eliminates the risk to the patient’s safety.
6,
7A version of the portable scanner is also available for intraoperative applications to assist the neurosurgeon in stunt placement, astrocytoma reduction, arteriovenous malformation resection, hematoma evacuation, and other procedures requiring checking for precision.
Advantages and Disadvantages
The advantages of CT scans are many. It is a painless, low-cost, widely available diagnostic tool that requires only minutes to complete, and can be performed on both conscious and unconscious patients. It is useful in rapidly developing neurological conditions when monitoring and life-support equipment is necessary. CT is sensitive to detect acute hemorrhage and calcifications and demonstrates the anatomy of skull and vertebral bones well. CT has drastically reduced the need for more dangerous and expensive diagnostic tests. It is also useful when MRI is contraindicated because of metal aneurysm clips, orthopedic prostheses, foreign objects in the eye, pacemakers, and other metal implants. Finally, the cost of a CT scan varies with the type and complexity of the study; generally it is about half the cost of an MRI.
8Some disadvantages are also noted. CT scans include ionizing radiation exposure about equal to that of skull films. By comparison, an MRI does not use radiation. CT scans have decreased sensitivity for detection of many common neurological diseases, lesions adjacent to bone, small soft tissue lesions, posterior fossa (cerebellum and brainstem) lesions, and floor of the middle fossa lesions.
3 Claustrophobia is a
contraindication for a CT scan. A patient who is unable to tolerate his or her head being in the confined space of a CT scanner is not a candidate for a CT scan.
Patient Preparation and Postprocedural Care
Screen for claustrophobia. All jewelry, eyeglasses, and hair clips or pins are removed from the head and neck. Unless a contrast medium will be administered, there are no dietary restrictions or other screening requirements before the procedure. The need to remain perfectly still is emphasized because movement can cause artifacts that will affect the clarity of the pictures. Agitated or uncooperative patients will require short-acting intravenous (IV) sedation such as lorazepam (Ativan) to ensure the necessary quality of the pictures.
Before the contrast medium is administered, a skin test may be done to check for an allergic reaction in high-risk patients. The blood urea nitrogen and creatinine levels are checked within 48 hours of the procedure to assess adequate kidney function. Most protocols require the patient to have had nothing by mouth for 4 to 8 hours before the test. Some patients experience flushing or a feeling of warmth, transient headache, a salty taste in the mouth, nausea, or vomiting when the contrast medium is administered; patients should be prepared for these possible reactions.
After the procedure, patients who have received contrast medium should be observed for signs and symptoms of delayed allergic reactions such as hives, skin rash, itching, nausea, headache, or seizures. If symptoms are severe, antihistamines may be ordered. IV hydration or oral fluid intake for hydration to facilitate clearance of contrast medium is very important. Tubular necrosis and acute kidney failure can result from inadequate hydration; older patients are at higher risk because of age-related kidney function changes. Monitor for both adequate intake and output.
Other Uses of Computed Tomography Technology in Diagnostics
CTA is emerging as an alternative to MRA for imaging extracranial and intracranial blood vessels. The CTA has several advantages over the MRA. It is faster (<32 seconds) than MRA and can be used with intubated patients or those with aneurysmal clips. It offers multiplanar views of the cerebral vasculature and visualization of slow flow or turbulent flow in aneurysms. Finally, it identifies plaque location and characteristics.
9 The limitations include risks associated with iodinated contrast media use and some difficulty in imaging the posterior inferior cerebellar arteries.
10Xenon-enhanced computed tomography (XeCT) evaluates cerebral blood flow (CBF). A mixture of oxygen and xenon is inhaled and the xenon serves as a contrast medium. The xenon gas rapidly dissolves in the blood and crosses the lipid-rich blood-brain barrier and enters the brain. The CBF is calculated using the modified Kety-Schmidt equation.
11 After breathing the xenon and oxygen mixture, the brain is scanned. Upon completion of the scanning, the xenon is rapidly washed from the cerebral tissue (half-life about 40 seconds).
Adverse reactions include nausea, reversible altered sensorium, or respiratory depression in less than 0.1 cases. Cerebrovascular disorders are the main clinical applications of XeCT, and it has been used to study cerebrovascular physiology. Currently, XeCT is useful to determine viability of cerebral tissue globally and regionally and can be used as part of brain death criteria.
Patient Preparation and Postprocedural Care
There is no special preparation or postprocedural care required other than monitoring for any adverse reaction.
Magnetic Resonance Imaging
The second major diagnostic tool for anatomic imaging of the brain and spinal column is MRI. It is a noninvasive, painless diagnostic procedure that provides better resolution slice images of anatomic structures of the brain and other soft tissue. The technology uses strong magnetic fields rather than ionized radiation, and thus requires special, copper-lined rooms for shielding from the strong
magnetic fields created by the MRI. Current technology uses hydrogen atoms (protons) because hydrogen is the most abundant element in tissue and yields the strongest magnetic signal. The presence of thermal energy causes each proton to spin like a top.
12MRI technology creates a strong magnetic field in which protons within the tissue align themselves in the longitudinal direction of the magnetic field.
8 A computer-programmed brief (few milliseconds) burst of
radiofrequency (RF) pulse is applied to the magnetic field, which changes the axis of the alignment of the atoms from the longitudinal to the transverse plane. This effect of rotating the atoms about the axes in a uniform manner is known as
resonance. When the RF is turned off, the atoms return to their original alignment, an effect known as
relaxation. The RF energy that was absorbed and then emitted results in an MR signal that is detected by electromagnetic receiver coils and then stored. Application of the RF is repeated several times as a
pulse sequence with the signals being measured and stored after each pulse application. Depending on how quickly these atoms return to their original state, the computer can determine which kind of tissue is represented by the returned signal. It is the
intensity of the returned signal reported as hypo-, iso-, or hyperintense that correlates to tissue type.
13 Computer programs analyze the stored data and an image is mathematically constructed from the combined data.
Table 6-2 provides basic MRI scan sequences.
The terms T1 and T2 refer to the weighted relaxation time constants for protons; these weights may be altered to highlight certain features of the tissue structure. In a
T1-weighted image, the CSF appears very dark, gray matter is lighter, and white matter is bright. The cortical border and cortical-white matter junctions are well defined. In a
T2-weighted image, the CSF appears white and there is poor color discrimination between gray and white matter. The T2 image highlights alterations in white matter such as infarction, demyelination, and edema.
3Pulse sequences refer to the different imaging techniques generated by manipulating the timing of the RF pulses. The pulse sequence variables, which are manipulated to produce different types of images, are known as echo time (TE) and repetition time (TR).
The type of image selected depends on the type of tissue under study and the suspected pathology. This ability to manipulate pulse sequence and components enhances the usefulness of MRI. Some of the more common pulse sequences include spin-echo, inversion recovery, gradient-echo, and fluid attenuation inversion recovery (FLAIR) sequences.
Table 6-3 includes special MRI sequences. Each type has characteristics that make it useful in special circumstances and sequences.
Gadolinium is the paramagnetic agent used as a contrast medium for MRI studies. It enhances the process of proton relaxation during the T1 sequence of MRI, resulting in sharper definition, and highlights the surrounding regions of lesions where the blood-brain barrier has been disrupted.
Clinical Applications
Because MRIs are not obscured by bone, the technique is excellent in detecting soft tissue changes that are insensitive with CT scan. These include ischemic and infarcted areas; degenerative diseases (e.g., multiple sclerosis, Alzheimer’s disease); cerebral and spinal cord edema; hemorrhage; arteriovenous malformations; small tumors, particularly in the difficult-to-visualize areas of the brainstem, basal skull, and spinal cord; and congenital anomalies. The superb sharpness and precision of detail are superior for diagnosis and location of lesions.