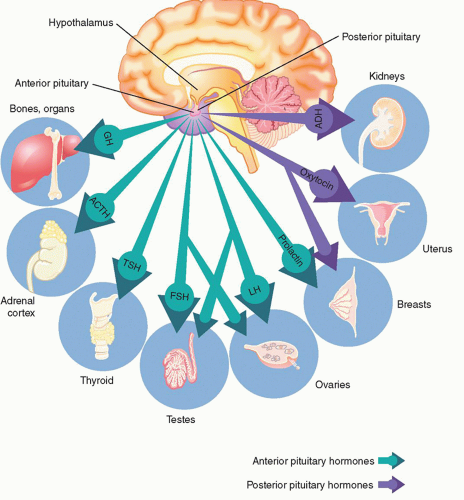
Adenohypophyseal Hormones
ACTH, regulated by CRH excreted from the hypothalamus, stimulates the production of glucocorticoids from the adrenal cortex. ACTH has a diurnal pattern of excretion and typically peaks just before awakening from sleep. Physical and emotional stress can increase production of ACTH. Alterations in ACTH affect the end production of cortisol. See Adrenal section for further discussion on the effects of alterations of cortisol.
Growth hormone (GH), regulated by the hypothalamus via GHRH and somatostatin, is responsible for linear growth in children, as well as metabolism and tissue integrity in both adults and children. GH levels can be impacted by several nonhypothalamic factors. Sleep deprivation can impact GH levels since 70% of the daily secretion of GH occurs during sleep stages 3 and 4. In addition, GH levels are impacted by dopamine. Dopamine agonists such as levodopa and bromocritpine increase GH levels whereas dopamine antagonists such as metoclopramide can decrease the levels. See
Table 10-1 for list of dopaminergic medications. Hypofunction may result from deficiency of GHRH or from a lack of GH receptors in the liver. Hypofunction is most commonly found in children and can result in dwarfism. Since intrauterine growth is independent of GH, children deficient in GH are normal size at birth and may not show signs of deficiency until adolescence. Hyperfunction, or excessive GH, in children where long bones are still growing can result in gigantism. In adults, long bone growth plates are inactive and excess GH instead leads to acromegaly. Acromegaly is most commonly associated with enlarged facial features, but can lead to serious cardiac arrhythmias.
Prolactin, responsible for lactation in the postpartum period is primarily inhibited by the hypothalamus through the neurotransmitter dopamine. Granulomas or pituitary adenomas compressing the pituitary stalk can prevent the free flow of dopamine down the hypophyseal portal veins resulting in hyperprolactinemia. Galactorrhea, or unexpected lactation, may be a presenting symptom in patients with pituitary adenoma, although several medications can result in hyperprolactinemia including those that affect dopamine levels.
Thyroid stimulating hormone (TSH) released from the adenohypophysis binds with the TSH receptors on the thyroid where they stimulate the thyroid gland to increase iodide uptake and release T3 and T4. TSH is regulated by both TRH and somatostatin via a negative feedback loop. Dopamine antagonists may increase TSH levels while dopamine agonists may have the opposite effect. In addition, octreotide, a somatostatin analog, can inhibit TSH as well. Primary hypothyroidism occurs due to a failure in the thyroid itself. Secondary hypothyroidism can occur as a result of hypothalamic or pituitary dysfunction. Patients with symptoms of hypothyroidism and a normal TSH level should be screened for primary hypothyroidism using T3 and T4 levels since the abnormality is occurring at the thyroid level and not the pituitary level.
Gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH), regulate gonadal function by promoting steroids responsible for sexual function and gametogenesis. LH excretion results in testosterone production in males and estrogen and progesterone in females. In males, FSH aids in maturation of spermatozoa, while in females promotes development of the ovarian follicle. Reproduction, while important to quality of life, is not an immediate concern for the neurological patient in the acute or rehabilitation phase and, therefore, will not be discussed in detail in this text.
Neurohypophyseal Hormones
Arginine vasopressin, also called
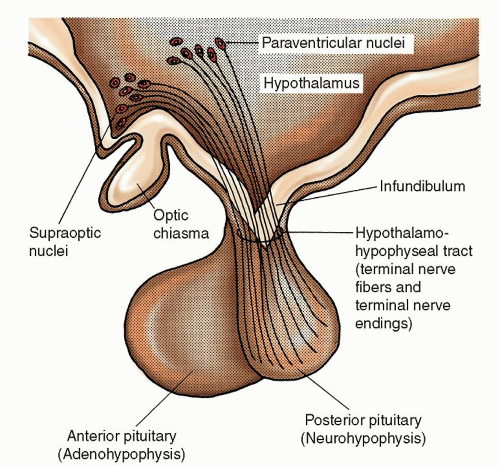
antidiuretic hormone (ADH), affects serum osmolality and total body water (TBW) by increasing water reabsorption in the collecting tubules of the kidney. The perikarya of the magnocellular neurons in the supraoptic and paraventricular nuclei synthesize most ADH. ADH then bonds loosely with a carrier protein called
neurophysin and is transported down the
pituitary stalk or
infundibulum through terminal nerve fibers and terminal nerve endings. Here ADH is stored in large secretory granules in the nerve endings of the posterior pituitary gland. Once the serum osmolality threshold of greater than 280 to 290 mOsmol/kg is reached, electrical impulses generated by the supraoptic and paraventricular nuclei control release of ADH from the nerve
endings. Because of the loose bonding of neurophysin to ADH, the neurophysin immediately separates from ADH, and adjacent capillaries absorb ADH releasing it into the bloodstream.
ADH is very potent, and even minute amounts (as little as 2 mcg) have an appreciable effect on water balance. The half-life of ADH is 15 to 20 minutes, with metabolic degradation occurring in the liver and kidney. Even minute changes in volume, concentration, and composition of body fluids respond quickly to ADH. The immediate release and rapid breakdown of ADH account for this quick response.
Three receptor sites exist for vasopressin, V1, V2, and V3. The V1 receptor activation causes vasoconstriction, while the V2 receptor is responsible for water reabsorption. The V3 receptor is the least understood but appears to play a role in the pituitary release of ACTH. Vasoconstriction through the activation of the V1 receptor is the primary use of the continuous infusion of intravenous (IV) vasopressin in patients with hypotension. Activation of the V2 receptor regulates ADH secretion maintaining serum osmolality in a narrow range by two major processes. As serum osmolality rises, ADH stimulates sodium chloride (NaCl) reabsorption in the thick ascending limb of the Loop of Henle while also causing water channels called aquaporins to open in the collecting ducts allowing water to be reabsorbed. Reabsorption of water normalizes serum osmolality, completing the feedback loop, and leading to a decreased secretion of ADH.
2 Conversely, without ADH present, the renal collecting ducts and tubules are almost totally impermeable to water so that fluid is not reabsorbed and is excreted in the urine. Desmopressin, a vasopressin analog, to be discussed in the section regarding DI may be administered to increase water reabsorption in the kidney. Desmopressin also increases factor VII and von Willebrand factor and is sometimes used to treat bleeding resulting from platelet dysfunction.
Certain conditions stimulate secretion of ADH, thereby conserving water in the body. They include the upright position, hyperthermia, hypotension, hypovolemia (especially caused by severe blood loss), pain, severe stress, anxiety, nausea, emesis, hypoxia, and trauma. Drugs that increase ADH release include acetaminophen (Tylenol), amitriptyline, anesthetic agents, angiotensin II agents, barbiturates, β-adrenergic agents, bromocriptine, chlorpromazine (Thorazine), chlorothiazide, cholinergic drugs, clofibrate (Atromid-S),
cyclophosphamide, haloperidol, histamines, monoamine oxidase inhibitors, meperidine hydrochloride, metoclopramide, morphine, nicotine, phenothiazines, prostaglandin E
2, and vincristine sulfate.
2,
3 Other conditions inhibit release of ADH. They include the recumbent position, hypothermia, hypertension, hypoosmolality, increased blood volume, and sleep. Drugs that decrease ADH secretion include ethanol, α-adrenergic agents, anticholinergic agents, demeclocycline, glucocorticosteroids (e.g., dexamethasone), lithium carbonate, narcotic antagonists, phenytoin, tolazamide, and vinblastine.
2,
3 Finally, chlorpropamide, carbamazepine, nonsteroidal anti-inflammatory drugs (NSAIDs), and tolbutamide enhance the effect of ADH.
Like vasopressin, oxytocin is synthesized in the magnocellular neurons of the supraoptic and paraventricular nuclei traveling down the infundibulum to be stored in the posterior pituitary. Oxytocin has two major targets, the mammary alveoli and the smooth muscle cells of the uterus. Responsible for species survival, oxytocin stimulates contractions of the uterus during the birthing process, stimulates milk ejection from the mammary glands in lactating women, and is thought to play a role in the bonding of mother to child.