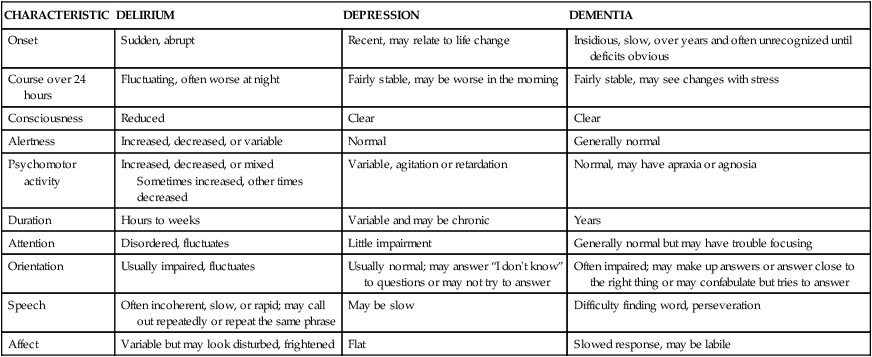
On completion of this chapter, the reader will be able to: 1. Discuss concepts related to cognition and memory in later life. 2. Differentiate between delirium, dementia, and depression. 3. Discuss nursing interventions for prevention and treatment of delirium. 4. Identify potential risk and modifying factors for the development of dementia. 5. Discuss the different types of dementia and appropriate diagnosis. 6. Describe nursing models of care for persons with dementia. 7. Discuss common concerns in care of persons with dementia and nursing responses. 8. Discuss strategies to enhance quality of life for caregivers of persons with dementia. 9. Develop a nursing care plan for an individual with cognitive impairment. We have artificially separated cognitive function from mental health, though they are in most ways interdependent. The mind is in some ways limited by the capacities of the brain, yet just as in medicine, there is a danger of evaluating the person by the measured and tested efficiency of cells and organs. Nowhere is this more important than in examining the cognition of older people. Citing John Morris, professor of neurology at Washington University in St. Louis, Crowley (1996) says that if brain function becomes impaired in old age, it is a result of disease, not aging. Cognitive health and health promotion activities to maintain brain health are discussed in Chapter 2; cognitive assessment instruments in Chapter 7; and communication with persons experiencing cognitive impairment in Chapter 6. Cognition is the process of acquiring, storing, sharing, and using information. Components of cognitive function include language, thought, memory, executive function, judgment, attention, and perception (Desai et al., 2010). The determination of intellectual capacity and performance has been the focus of a major portion of gerontological research. Cognitive functions may remain stable, or decline with increasing age. The cognitive functions that remain stable include attention span, language skills, communication skills, comprehension and discourse, and visual perception. The cognitive skills that decline are verbal fluency, logical analysis, selective attention, object naming, and complex visuospatial skills. High CR may allow the individual to continue to learn and adapt to changing stimuli, despite the presence of age-related changes. CR is probably set early in the first two to three decades of life, and stimulating environments, education, and healthy lifestyles throughout life appear to enhance cognitive reserve. CR affects the ability of the adult brain to sustain normal function in the face of significant disease or injury. Brain diseases and injuries may be less apparent in those with greater CR because they are able to tolerate lost neurons and synapses (Desai et al., 2010; Yevchak et al., 2008). For example, individuals who attained more years of education may have high levels of Alzheimer’s disease pathology, but few, if any, clinical symptoms. There are many myths about aging and the brain that may be believed by both health professionals and older adults. It is important to understand cognition and memory in late life and dispel the myths that can have a negative effect on wellness and may, in fact, contribute to unnecessary cognitive decline (Box 19-1). The cognitive development of older people is often measured against the norms of young or middle-aged people, which may not be appropriate to the distinctive characteristics of older adults. In addition, most tests of cognitive ability were designed to test young children, and most do not address cultural or ethnic differences. Other reasons have been advanced for the variations of intellectual performance of the older adult being tested (Box 19-2). Therefore, these tests may have little relevance for the daily function of older people. Intelligence in old age is dynamic, and certain abilities change and even improve with age. Fluid intelligence (often called native intelligence) consists of skills that are biologically determined, independent of experience or learning. It is associated with flexibility in thinking, inductive reasoning, abstract thinking, and integration. Fluid intelligence “enables people to identify and draw conclusions about complex relationships” (Miller, 2008, p. 187). Crystallized intelligence is composed of knowledge and abilities that the person acquires through education and life. Measures of crystallized intelligence include verbal meaning, word association, social judgment, and number skills. Older people perform more poorly on performance scales (fluid intelligence), but scores on verbal scales (crystallized intelligence) remain stable. This is known as the classic aging pattern (Hooyman and Kiyak, 2011). The tendency to do poorly on performance tasks may be related to age-related changes in sensory and perceptual abilities as well as psychomotor skills. Speed of cognitive processing and slower reaction time also affect performance. Late adulthood is no longer seen as a period when growth has ceased and cognitive development halted; rather it is seen as a life stage programmed for plasticity and the development of unique capacities. Older people do maintain their ability to understand situations and learn from new experiences. These findings are significant to satisfaction in late life, because the capacity for effective lifestyle management and one’s cognitive resources contribute to adaptation and enjoyment. Chapter 6 discusses learning in later life and presents effective teaching-learning strategies. Cognitive stimulation and memory training may be helpful for cognitively intact older adults, as well as for those with cognitive impairment (Camp and Skrajner, 2004; Yevchak et al., 2008). Cognitive stimulation and memory training techniques include mnemonics (strategies to enhance coding, storage, and recall), internal and external aids, reasoning and speed-of-processing training, cognitive games (e.g., Scrabble, chess, crossword puzzles), and spaced retrieval techniques. Many games and aids are available that may be useful to enhance memory and stimulate cognitive function. Continued attention to diet, exercise, cardiovascular risk factors, and meaningful activities are also important to brain health (Chapter 2 and Table 19-3). An older person with a change in cognitive function needs a thorough assessment to identify the presence of specific pathological conditions. Pathological conditions causing impairment of cognition include delirium, dementia, and depression. The literature reveals that both physicians and nurses in all settings routinely fail to appropriately assess an individual’s cognitive functioning. Pathological conditions are often undiagnosed, reversible causes not identified, and opportunities for early intervention missed. As a result, the person experiences greater impairment and functional decline (Braes et al., 2008). Some of the reasons for this include the complexity of cognitive assessment and the existence of several conditions with similar symptoms (dementia, depression, delirium) (Table 19-1). Other reasons include the often atypical symptom presentation in older adults and the belief, on the part of health care professionals as well as older people, that alterations in cognitive functioning are part of the “normal” aspects of aging (Braes et al., 2008; Evans, 2007; Fletcher, 2008). Older people may be diagnosed with dementia as a result of an occurrence of confusion or altered mental status without a comprehensive evaluation. Older adults should be routinely and regularly assessed for cognitive function in all settings, and nurses must have the skills to recognize cognitive impairment and monitor cognitive functioning. “Assessment of cognitive function is the first and most critical step in a cascade of strategies to prevent, reverse, halt, or minimize cognitive decline” (Braes et al., 2008, p. 42). (Chapter 7 discusses assessment tools used to evaluate cognitive function). TABLE 19-1 DIFFERENTIATING DELIRIUM, DEPRESSION, AND DEMENTIA Modified from Sendelbach S, Guthrie PF, Schoenfelder DP: Acute confusion/delirium, J Gerontol Nurs 35(11):11-18, 2009. Dementia, delirium, and depression have been called the three D’s of cognitive impairment because they occur frequently in older adults. These important geriatric syndromes are not a normal consequence of aging, although incidence increases with age. Because cognitive and behavioral changes characterize all three D’s, it can be difficult to diagnose delirium, delirium superimposed on dementia (DSD), or depression. Inability to concentrate, with resulting memory impairment and other cognitive dysfunction, can occur in late-life depression. The term pseudodementia has been used to describe the cognitive impairment that may accompany depression in older adults (Chapter 18). Delirium is characterized by an acute or subacute onset, with symptoms developing over a short period of time (usually hours to days). Symptoms tend to fluctuate over the course of the day, often worsening at night. Symptoms include disturbances in consciousness and attention and changes in cognition (memory deficits, perceptual disturbances). Perceptual disturbances are often accompanied by delusional (paranoid) thoughts and behavior (Evans and Kurlowicz, 2007). In contrast, dementia typically has a gradual onset and a slow, steady pattern of decline without alterations in consciousness (Voyer et al., 2010). Knowledge about cognitive function in aging and appropriate assessment and evaluation are keys to differentiating these three syndromes. Table 19-1 presents the clinical features and the differences in cognitive and behavioral characteristics in delirium, dementia, and depression. The accepted criteria for a diagnosis of delirium are presented in the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatic Association, 2000). The development of delirium is a result of complex interactions among multiple causes. Delirium results from the interaction of predisposing factors (e.g., vulnerability on the part of the individual due to predisposing conditions, such as cognitive impairment, severe illness, and sensory impairment) and precipitating factors/insults (e.g., medications, procedures, restraints, iatrogenic events). While a single factor, such as an infection, can trigger an episode of delirium, several co-existing factors are also likely to be present. A highly vulnerable older individual requires a lesser amount of precipitating factors to develop delirium (Inouye et al., 1999; Voyer et al., 2010). The exact pathophysiological mechanisms involved in the development and progression of delirium remain uncertain, and further research is needed to understand its neuropathogenesis. Delirium is thought to be related to disturbances in the neurotransmitters in the brain that modulate the control of cognitive function, behavior, and mood. Irving and Foreman (2006) note that “there is growing evidence of cholinergic failure as a common pathway in delirium” (p. 122). The causes of delirium are potentially reversible; therefore accurate assessment and diagnosis are critical. Delirium is given many labels: acute confusional state, acute brain syndrome, confusion, reversible dementia, metabolic encephalopathy, and toxic psychosis. Delirium is a prevalent and serious disorder that occurs in elders across the continuum of care. Estimates are that delirium may affect up to 42% of hospitalized older adults and as many as 87% of older adults in intensive care units (ICUs) (Cole and McCusker, 2009; Marcantonio et al., 2010; Sweeny et al., 2008). Older people who have undergone surgery and those with dementia are particularly vulnerable to delirium. The prevalence of delirium is as high as 65% after orthopedic surgery, particularly hip fracture repair (Rigney, 2006). Among older people experiencing cardiac surgery, as many as 20% to 25% experience delirium, affecting even those without any documented preoperative cognitive impairments (Clarke et al., 2010). A 16% delirium rate in patients newly admitted to subacute care has been reported. More than 50% of these patients are still delirious one month after admission (Marcantonio et al., 2010). Delirium is associated with increased morbidity, mortality, and institutionalization, independent of age, co-existing illnesses, or illness severity (Witlox et al., 2010). The incidence of delirium superimposed on dementia (DSD) ranges from 22% to 89% (Tullmann et al., 2008). Older patients with dementia are three to five times more likely to develop delirium, and it is less likely to be recognized and treated than is delirium without dementia. DSD is associated with high mortality among hospitalized older people (Bellelli et al., 2008). Changes in the mental status of older people with dementia are often attributed to underlying dementia, or “sundowning,” and not investigated. This is particularly significant since about 25% of all older hospitalized patients may have Alzheimer’s disease or another dementia (Voelker, 2008). Delirium can accelerate the trajectory of cognitive decline in individuals with Alzheimer’s disease. Further research is needed to determine whether prevention of delirium may delay cognitive decline in individuals with Alzheimer’s disease (Fong et al., 2009). Despite its prevalence, DSD has not been well investigated, and there are only a few relevant studies in either the hospital or community setting. Delirium is a medical emergency and one of the most significant geriatric syndromes (Waszynski and Petrovic, 2008). However, it is often not recognized by physicians or nurses. Studies indicate that delirium is unrecognized in 66% to 84% of patients (Pisani et al., 2006; Balas et al., 2007). A comprehensive review of the literature suggested that “nurses are missing key symptoms of delirium and appear to be doing superficial mental status assessments” (Steis and Fick, 2008, p. 47). Factors contributing to the lack of recognition of delirium among health care professionals include inadequate education about delirium, a lack of formal assessment methods, a view that delirium is not as essential to the patient’s well-being in light of more serious medical problems, and ageist attitudes (Kuehn, 2010a; Waszynski and Petrovic, 2008). Failure to recognize delirium, identify the underlying causes, and implement timely interventions contributes to the negative sequelae associated with the condition (Tullmann et al., 2008; Kuehn, 2010a). Dahlke and Phinney (2008) investigated interventions nurses use to assess, prevent, and treat delirium, as well as the challenges and barriers nurses face in caring for patients with delirium in the acute care setting. The authors concluded that cognitive changes in older people are often labeled confusion by nurses and physicians, are frequently accepted as part of normal aging, and are rarely questioned. If the nurse believed that confusion was normal in older adults, he or she would be less likely to recognize symptoms of delirium as a medical emergency necessitating their attention and intervention. Confusion in a child or younger adult would be recognized as a medical emergency, but confusion in older adults may be accepted as a natural occurrence, “part of the older person’s personality” (p. 46). In the Dahlke and Phinney study, nurses reported that caring for patients with delirium was seen as “annoying, frustrating and not interesting” (2008, p. 45). Nurses expressed that the care of older patients with delirium interfered with what was perceived as the “real work” of caring for a medical or surgical patient. Insufficient knowledge and inadequate time and resources also influenced appropriate care. The authors conclude that nurses are faced with the predicament of fitting care for older adults into a system that does not recognize the unique needs of this population. Clearly, education and attitudes about older people must be addressed if we want to improve care outcomes for the growing number of older adults who will need care. The risk of delirium increases with the number of risk factors present. The more vulnerable the individual, the greater the risk. The multifactorial model for delirium (MMD), developed by Inouye and Charpentier (1996), can be used to guide identification of risk factors in hospitalized older adults. In the first study examining the use of this model in long-term care, Voyer and colleagues (2010) report that the MMD is relevant among this population and can be used to prevent delirium and improve care outcomes in this setting. Identification of high-risk patients, risk factors, prompt and appropriate assessment, and continued surveillance are the cornerstones of delirium prevention. More than 35 potential risk factors have been identified for delirium. Among the most predictive are immobility, functional deficits, use of restraints or catheters, medications, acute illness, infections, alcohol or drug abuse, sensory impairments, malnutrition, dehydration, respiratory insufficiency, surgery, and cognitive impairment. Unrelieved or inadequately treated pain significantly increases the risk of delirium (Irving and Foreman, 2006). Medications account for 22% to 39% of all delirium, and all medications, particularly those with anticholinergic effects and any new medications, should be considered suspect. Invasive equipment, such as nasogastric tubes, intravenous (IV) lines, catheters, and restraints, also contribute to delirium by interfering with normal feedback mechanisms of the body (Box 19-3). Delirium is categorized according to the level of alertness and psychomotor activity. The clinical subtypes are hyperactive, hypoactive, and mixed. Box 19-4 presents the characteristics of each of these clinical subtypes. In non-ICU settings, approximately 30% of delirium is hyperactive, 24% hypoactive, and 46% is mixed. Because of the increased severity of illness and the use of psychoactive medications, hypoactive delirium may be more prevalent in the ICU. Although the negative consequences of hyperactive delirium are serious, the hypoactive subtype may be missed more often and is associated with a worse prognosis because of the development of complications such as aspiration, pulmonary embolism, pressure ulcers, and pneumonia. Increased hospital stays, longer duration of delirium, and higher mortality have been associated with hypoactive delirium. Delirium has serious consequences and is a “high priority nursing challenge for all nurses who care for older adults” (Tullmann et al., 2008, p. 113). Delirium results in significant distress for the patient, his or her family and significant others, and nurses. Delirium is associated with increased length of hospital stay and hospital readmissions, increased services after discharge, and increased morbidity, mortality, and institutionalization, independent of age, co-existing illnesses, or illness severity (Witlox et al., 2010). Recent research indicates that delirium is associated with lasting cognitive impairment and psychiatric problems. While the majority of hospital inpatients recover fully from delirium, a substantial minority will never recover or recover only partially. Patients with delirium that remains unresolved at discharge should be screened again at three months and followed closely. The persistence of delirium after discharge may interfere with the ability to manage chronic conditions and contribute to poor outcomes (Cole and McCusker, 2009). Screening all older adults before they leave the hospital may help to identify those in need of specific transitional care (Chapter 16) with more frequent follow up after hospitalization (Lindquest et al., 2011). Further research is needed to determine the reasons for the long-term poor outcomes, whether characteristics of the delirium itself (subtype or duration) influence prognosis, and how the long-term effects might be decreased. Several instruments can be used to assess the presence and severity of delirium. To detect changes, it is very important to determine the person’s usual mental status. If the person cannot tell you this, family members or other caregivers who are with the patient can be asked to provide this information. If the patient is alone, the responsible party or the institution transferring the patient can provide this information by phone. Do not assume the person’s current mental status represents his or her usual state, and do not attribute altered mental status to age alone or assume that dementia is present. All older patients, regardless of their current cognitive function, should have a formal assessment to identify possible delirium when admitted to the hospital (Box 19-5). The MMSE-2 is considered a general test of cognitive status that helps identify mental status impairment. Although the MMSE-2 alone is not adequate for diagnosing delirium, it represents a brief, standardized method to assess mental status and can provide a baseline from which to track changes (see also Chapter 7). Several delirium-specific assessment instruments are available, such as the Confusion Assessment Method (CAM) (Inouye et al., 1990) and the NEECHAM Confusion Scale (Neelon et al., 1996). The CAM-ICU is another instrument specifically designed to assess delirium in an intensive care population and has recently been validated for use in critically ill, nonverbal patients who are on mechanical ventilation (Ely et al., 2001; Rigney, 2006). Assessment using the MMSE-2, CAM, and NEECHAM should be conducted on admission to the hospital, throughout the hospitalization for all patients identified at risk for delirium, and for all patients who exhibit signs and symptoms of delirium or develop additional risk factors (Steis and Fick, 2008). Results of a study (Waszynski and Petrovic, 2008) suggested that the CAM was useful in identifying delirium in hospitalized adults, and nurses found it very helpful in identifying changes in cognitive functioning. As a result of these findings, the CAM was made a customary part of the daily flow sheet. Intervention begins with prevention. An awareness and identification of the risk factors for delirium and a formal assessment of mental status are the first-line interventions for prevention. Balas and colleagues (2007) suggest that “nurses make multiple decisions throughout the day that can potentially enhance or diminish the likelihood that their patients will experience delirium” (p. 152). Nurses play a pivotal role in the identification of delirium, and it is imperative that they accurately report patients’ mental status to the medical team so that causative factors can be identified and treated (Irving and Foreman, 2006). Because the etiology of delirium is multifactorial, “for an intervention strategy to be effective, it should target the multifactorial origins of delirium with multicomponent interventions that address more than one risk factor” (Rosenbloom-Brunton et al., 2010, p. 23). Multidisciplinary approaches to prevention of delirium seem to show the most promising results, but continued research is needed to evaluate what type of approach has the most beneficial effect in specific clinical settings. The current disease model of delirium care is not effective (Dahlke and Phinney, 2008). A well-researched multidisciplinary program of delirium prevention in the acute care setting, the Hospital Elder Life Program (HELP) (Inouye et al., 1999; Bradley et al., 2005; Rubin et al., 2006), focuses on managing six risk factors for delirium: cognitive impairment, sleep deprivation, immobility, visual impairments, hearing impairments, and dehydration. The program is used in more than 60 hospitals in the United States and internationally. Patient outcomes with the use of this model include a 40% reduction in the incidence of delirium, a 67% reduction in rates of functional decline, and significant cost savings in both hospitals and long-term care facilities. Most of the interventions can be considered quite simple and part of good nursing care. The Family-HELP program, an adaptation and extension of the original HELP program, trains family caregivers in selected protocols (e.g., orientation, therapeutic activities, vision and hearing). Initial research demonstrates that active engagement of family caregivers in preventive interventions for delirium is feasible and supports a culture of family-oriented care (Rosenbloom-Brunton et al., 2010). Examples of interventions in the HELP program include the following: offering herbal tea or warm milk instead of sleeping medications, keeping the ward quiet at night by using vibrating beepers instead of paging systems, using silent pill crushers, removing catheters and other devices that hamper movement as soon as possible, encouraging mobilization, assessing and managing pain, and correcting hearing and vision deficits. Fall risk reduction interventions, such as bed and chair alarms, low beds, reclining chairs, volunteers to sit with restless patients, and keeping routines as normal as possible with consistent caregivers, are other examples of interventions. Further information on the Elder Life Program can be found at http://elderlife.med.yale.edu/public/public-main.php. Box 19-6 presents suggested interventions for delirium. Another innovative approach is the delirium doula. Borrowing the concept of a “doula” from maternity care, this concept was designed by student nurses who had completed a maternity placement where doulas were used. The proposed role of the delirium doula would include providing support, adjusting the environment to meet the patient’s behavior or needs, and assisting the patient to get help when required (Balas et al., 2004; Balas et al., 2007; Irving and Foreman, 2006; Sweeny et al., 2008). A commonly used intervention for patients with delirium in acute care is the use of “sitters” or “constant observers (COs).” Costs associated with this practice can be very high, and data indicate that the use of sitters or COs does not consistently decrease the incidence of unsafe patient behavior in the patient with delirium. Nor do they assist in identifying causes of delirium or identifying appropriate interventions. Sweeny and colleagues (2008) report on the implementation of a multicomponent, evidence-based alternative to COs for care of patients with delirium in an acute care hospital. The program focused on fall risk–reduction strategies, as well as assessment of delirium using the CAM, and a protocol for intervention. Results suggest that costs associated with COs decreased from $1.5 million to $250,000 in 2 years with no change in the use of restraints or the incidence of falls. Pharmacological interventions to treat the symptoms of delirium may be necessary if patients are in danger of harming themselves or others, or if nonpharmacological interventions are not effective. However, pharmacological interventions should not replace thoughtful and careful evaluation and management of the underlying causes of delirium. Pharmacological treatment should be one approach in a multicomponent program of prevention and treatment. Research on the pharmacological management of delirium is limited, but it has been suggested that “with increased understanding of the neuropathogenesis of delirium, drug therapy could become primary to the treatment of delirium” (Irving and Foreman, 2006, p. 122). A few studies have suggested that use of dexmedetomidine as a sedative or analgesic may reduce the incidence or duration of delirium (Kuehn, 2010b). Ozbolt and colleagues (2008) conducted a literature review of the use of antipsychotics for treatment of delirious elders and concluded that the atypical antipsychotics demonstrate similar rates of efficacy to haloperidol for the treatment of delirium and have a lower rate of extrapyramidal side effects. Further research is needed since no double-blind placebo trials exist. Short-acting benzodiazepines are often used to control agitation but may worsen mental status. In ICU patients with delirium, lorazepam has been identified as an independent risk factor for developing delirium (Pandharipande et al., 2006). Psychoactive medications, if used, should be given at the lowest effective dose, monitored closely, and reduced or eliminated as soon as possible so that recovery can be assessed. Caring for patients with delirium can be a challenging experience. Patients with delirium can be difficult to communicate with, and disturbing behaviors such as pulling out IV lines or attempting to get out of bed disrupt medical treatment and compromise safety. It is important for nurses to realize that behavior is an attempt to communicate something and express needs. The patient with delirium feels frightened and out of control. The calmer and more reassuring the nurse is, the safer the patient will feel. Box 19-7 presents some communication strategies that are helpful in caring for people experiencing delirium. In contrast to delirium, which is usually a reflection of an acute physiological disturbance or severe depression that may affect cognition, dementia is an irreversible state that progresses over years and causes memory impairment and loss of other intellectual abilities severe enough to cause interference with daily life. Degenerative dementias include Alzheimer’s disease (AD), Parkinson’s disease dementia (PDD), dementia with Lewy bodies (DLB), and frontotemporal lobe dementias (FTDs). Alzheimer’s disease (AD) accounts for 50% to 70% of all dementia cases. Vascular cognitive impairment (VCI) encompasses several syndromes: vascular dementia; mixed primary neurodegenerative disease and vascular dementia; and cognitive impairment of vascular origin that does not meet the dementia criteria. Increasing evidence suggests that most dementias have neurodegenerative (most commonly AD) and vascular features, and these seem to act synergistically (Desai et al., 2010). Other less commonly occurring dementias are Creutzfeldt-Jakob disease (CJD) (subacute spongiform encephalopathy); and human immunodeficiency virus (HIV)–related dementia. Normal pressure hydrocephalus (NPH) causes a dementia characterized by ataxic gait, incontinence, and memory impairment. This disease is reversible and treated with a shunt that diverts cerebrospinal fluid away from the brain (Alzheimer’s Association, 2010a; Desai et al., 2010; Kamat et al., 2010) (Table 19-2). TABLE 19-2 TYPES OF DEMENTIA AND TYPICAL CHARACTERISTICS
Cognitive Impairment
![]() http://evolve.elsevier.com/Ebersole/TwdHlthAging
http://evolve.elsevier.com/Ebersole/TwdHlthAging
Adult Cognition
Fluid and Crystallized Intelligence
Memory
Cognitive Assessment
CHARACTERISTIC
DELIRIUM
DEPRESSION
DEMENTIA
Onset
Sudden, abrupt
Recent, may relate to life change
Insidious, slow, over years and often unrecognized until deficits obvious
Course over 24 hours
Fluctuating, often worse at night
Fairly stable, may be worse in the morning
Fairly stable, may see changes with stress
Consciousness
Reduced
Clear
Clear
Alertness
Increased, decreased, or variable
Normal
Generally normal
Psychomotor activity
Increased, decreased, or mixed
Sometimes increased, other times decreased
Variable, agitation or retardation
Normal, may have apraxia or agnosia
Duration
Hours to weeks
Variable and may be chronic
Years
Attention
Disordered, fluctuates
Little impairment
Generally normal but may have trouble focusing
Orientation
Usually impaired, fluctuates
Usually normal; may answer “I don’t know” to questions or may not try to answer
Often impaired; may make up answers or answer close to the right thing or may confabulate but tries to answer
Speech
Often incoherent, slow, or rapid; may call out repeatedly or repeat the same phrase
May be slow
Difficulty finding word, perseveration
Affect
Variable but may look disturbed, frightened
Flat
Slowed response, may be labile
Delirium
Etiology
Incidence and Prevalence
Recognition of Delirium
Risk Factors for Delirium
Clinical Subtypes of Delirium
Consequences of Delirium
Promoting Healthy Aging: Implications for Gerontological Nursing
Assessment
Interventions
Nonpharmacological
Pharmacological
Dementia



