Chapter 35 Physiological changes from late pregnancy until the onset of lactation
After reading this chapter, you will be able to
Introduction
Over the last 4 weeks of pregnancy, all women experience significant emotional and cognitive changes associated with ‘nesting’ and other signs of the emergence of maternal responsiveness and affiliation (Brunton & Russell 2008, Grattan 2002, Neumann 2009). At the same time, maternal pain threshold rises, the hypothalamic–pituitary axis (HPA) becomes hyporesponsive to various stressors, and women often experience periods of heightened apprehension relating to fetal and neonatal wellbeing (Douglas 2010, Douglas et al 2005, Gintzler & Liu 2001, Kask et al 2008, Neumann 2009, Russell & Brunton 2006).
During late pregnancy and lactation, anxiety behaviours in response to stressful stimuli are attenuated by central oxytocin and prolactin, while aggressive defensive behaviours and reduced fearfulness in response to perceived threats to the fetus and neonate increase from late pregnancy to advanced labour and peak during lactation (Kinsley 2008, Neumann 2009) (see website). Several neurotransmitters and neuropeptides are implicated in regulating maternal aggression, including local release of oxytocin within selected areas of the hypothalamus during and after birth (Brunton & Russell 2008, Neumann 2009, Russell & Brunton 2009).
Current findings on the affective and behavioural manifestations of these altered patterns of neuronal interactions provide insights into the sensibilities and vulnerabilities of mothers, before, during and after birth (Neumann 2009). A variety of studies indicate that maternal and neonatal outcomes are positively influenced by women’s chosen place of birth; emotional security and seclusion during labour; vaginal birth; and undisturbed maternal–infant sensory contact, particularly during the first hours of extrauterine life (DeVries 2002, Erlandsson & Fagerberg 2005, Hodnett et al 2008, Johnson & Davis 2005, Klein et al 1995, Swain et al 2008).
Acquiring brain capacities for mothering
Maternal HPA axis, social aggression and anxiety-related behaviours
Evidence from human and animal studies indicate that pregnancy and lactation are characterized by an enhanced parasympathetic tone, suppression of sympathetic responses to stressful stimuli, and attenuated response of the hypothalamic–pituitary–adrenal (HPA) axis to various stressors (Brunton et al 2008, Douglas 2010). This is manifested by the woman’s different responses to stressors and perceived dangers to herself and her baby. A key regulator of maternal anxiety postpartum appears to be physical separation of mother and infant (see website).
Prolactin – maternal stress and anxiety
In humans, levels of prolactin and hPL rise throughout pregnancy. During advanced labour, prolactin levels decline rapidly but rise again soon after birth (see website). Following placental separation, hPL disappears from the maternal circulation, but suckling provides the stimulus for ongoing prolactin secretion that peaks within the first 3 months of human lactation (Diaz et al 1989, Grattan 2002).
Prolactin has been found to initiate key elements in the repertoire of behaviours involved in nest-building during late pregnancy and nurturing, protecting and nourishing the infant during lactation (Lucas et al 1998): reduction of maternal fearfulness and anxiety, and reduced anxiety and stress during lactation (see website).
The maternal emotional brain – oxytocin and prolactin
In pregnancy, magnocellular oxytocin neurons in the supraoptic nucleus (SON) and paraventricular nucleus (PVN) are restrained from premature activation, to prevent pre-term labour and preserve accumulating oxytocin stores in the neurohypophysis, in preparation for labour, birth and the onset of lactation (Higuchi & Okere 2002, Russell & Brunton 2006, 2009, Russell et al 2003) (see website).
Gestational analgesia
A significant rise in maternal pain threshold occurs between late pregnancy and 24 hours following birth (Gintzler & Liu 2001, Whipple et al 1990). Maternal pain threshold rises gradually from 30 weeks’ gestation, accelerates during the last 3–4 weeks of pregnancy, rises further during labour, and then falls precipitously within 24 hours of birth (Gintzler & Liu 2001, Ohel et al 2007). Evidence indicates that placental steroids augment pelvic afferent tone. These nerves entering the spinal cord from the cervix and uterus activate multiple analgesic synergies between spinal κ/δ opioid systems, non-opioid peptides and spinal noradrenergic pathways descending from the brainstem (Liu & Gintzler 2003) (see website).
Painless nocturnal contractions occur in women from around 30 weeks’ gestation, coinciding with higher circulating levels of oxytocin and melatonin, and a lower oestrogen/progesterone ratio, which increases myometrial sensitivity to oxytocin during the early hours of darkness (Fuchs et al 1992, Murphy Goodwin 1999, Sharkey et al 2009).
Neuroendocrine and central oxytocin systems – late pregnancy to weaning
During pregnancy, secretion of oxytocin and secretory response of magnocellular neurons to various physiological stimuli are progressively restrained at several levels by opioid systems which are largely stimulated by allopregnanolone – a neurosteroid metabolite of progesterone (Brunton & Russell 2008, Douglas 2010, Higuchi & Okere 2002, Russell & Brunton 2009). In late pregnancy, brainstem and forebrain neuronal projections to the SON and PVN are activated in preparation for labour, birth, the induction of maternal behaviours and lactation (de Kock et al 2003, Douglas et al 2002, Ortiz-Miranda et al 2005; Russell et al 2003) (see website).
Central oxytocin
A distinct and independently regulated oxytocin system exists within the brain which is highly activated during the peripartum period. Central oxytocin acts as a neurotransmitter within PVN neurons that project to the forebrain, limbic system and autonomic centres in the brainstem and spinal cord (Neumann 2009, Russell & Brunton 2009). Oxytocin is also released in much larger quantities from soma and dendrites of magnocellular neurons within the SON, PVN and other associated nuclei throughout labour and lactation (Russell et al 2003) (see website).
Myometrial quiescence
Throughout pregnancy, the smooth muscle of the myometrium undergoes a series of adaptations that facilitate proliferation and hypertrophy while the capacity for contractility is deactivated (Shynlova et al 2009). This state of quiescence facilitates implantation, placental formation, subsequent growth of the fetus and placenta, and the progressive accumulation of amniotic fluid (Price & Lopez Bernal 2001).
An array of factors maintain uterine quiescence until the end of pregnancy, including human chorionic gonadotrophin (hCG), progesterone, corticotrophin-releasing hormone (CRH), relaxin, nitric oxide and melatonin. hCG inhibits formation of gap junctions; oxytocin stimulates contractions and stimulates enzymes that synthesize relaxatory prostaglandins, until receptor concentrations for hCG decline with the onset of labour (Sparey et al 1999, Ticconi et al 2006, Zuo et al 1994).
Localized myometrial contractions occur spontaneously in response to uterine distension, towards the end of pregnancy, when uterine growth declines relative to the fetus (Shynlova et al 2009). This increases uterine wall stretch, facilitating a pre-labour rise in myometrial oxytocin receptors. Prostacyclin increases the expression of gap-junction and contractile proteins, and melatonin receptor expression declines relative to non-pregnant values, attenuating its suppressive effects on myometrial oxytocin receptors (Fetalvero et al 2008, Lindstrom & Bennett 2005, Terzidou et al 2005).
Placental steroids
Plasma concentrations of oestrogens and progesterone increase progressively throughout pregnancy and labour, but target tissue responsiveness is controlled by changes in expression and activation of their nuclear and non-nuclear receptor subtypes, and by pregnancy-induced expression of progesterone metabolites, which maintain uterine quiescence by binding directly to membrane-bound receptors and inhibiting signalling pathways (Condon et al 2003, Mesiano 2001, Mesiano et al 2002, Mesiano & Welsh 2007, Sheehan et al 2005). The capacity of progesterone to maintain uterine quiescence is also enhanced by functional inactivation of sympathetic nerves in the myometrium and increased receptors for peptides and neurotransmitters that promote relaxation and inhibit the contractile effects of oxytocin (Casey et al 1997, Dong et al 1999, 2003, Ferguson et al 1998, Grammatopoulos et al 1996, Owman 1981, Price & Lopes Bernal 2001) (see website).
This complex balance between the oestrogen and progesterone ratios ensures that there is less myometrial responsiveness, desensitizing it to oestrogen-induced formation of gap junctions, contraction-associated proteins and responsiveness to oxytocin. At the same time, oestrogens increase myometrial responsiveness to the progesterone receptor, subtype B (PR-B) (Mesiano 2001, Mesiano et al 2002, Mesiano & Welsh 2007) (see website). Progesterone contributes to myometrial quiescence by modulating the expression of genes that encode a number of contraction-associated proteins, including oxytocin receptors and formation of myometrial gap-junctions, until the end of pregnancy (Mesiano et al 2002, Mesiano & Welsh 2007).
Placenta and fetal membranes
During pregnancy, spontaneous, oxytocin- and prostaglandin-induced myometrial contractions are inhibited by the placenta and chorioamniotic membranes surrounding the uterus. The placenta produces atrial natriuretic peptide (ANP), while the chorion and amnion produce brain natriuretic peptide (BNP). Both peptides inhibit oxytocin-induced contractions (Carvajal et al 2006, 2009; Cootauco et al 2008). These are ideally positioned to protect the fetus from oxytocin and other inflammatory mediators, with the capacity to stimulate myometrial contractility (Keelan et al 2003) (see website).
Amnion, chorion and decidua also express enzymes to synthesize and metabolize oestrogens and progesterone. Current evidence suggests that the dominance of PR-B is maintained in these tissues until the end of pregnancy, when enzymatic changes stimulate concurrent increases in the most biologically active oestrogen and the most inactive progestogen (Blanks et al 2003). The fetoplacental membranes and maternal decidual tissues therefore establish endocrine–paracrine networks regulating the length of gestation and the onset of labour (Chibbar et al 1995, Cootauco et al 2008, Henderson & Wilson 2001, Jaffe 2001, Rehman et al 2007, Smith 2007, Ticconi et al 2006).
From pregnancy to labour
Fetal preparations for labour and lactation
The progressive nocturnal rhythm in uterine activity during the last trimester gradually shifts the fetus towards the lower pole of the uterus and the presenting part descends into the pelvis. This helps the fetus to increase flexion and descent, and follow the curve of Carus. Activation of the fetal HPA axis during the third trimester produces physiological increases in cortisol, which interacts with other hormones to induce maturational changes in organs like the lungs, liver, pancreas and gut, thyroid axis, and thermogenic proteins in brown adipose tissue (Fowden et al 1995, Freemark 1999, Garbrecht et al 2006, Liggins 1994). In the brain, catecholaminergic neurons have a key role in cortical differentiation and maturation of respiratory neural networks in the brainstem (Fujii et al 2006). Cortisol and adrenaline also stimulate a gradual increase in blood pressure, in preparation for pulmonary expansion and cessation of the fetoplacental circulation soon after birth (see website).
During the last couple of days before the onset of labour, fetal breathing activity is reduced and lung fluid is produced at a gradually decreasing rate (Bland 2001). Fetal breathing may be depressed by endogenous opioids and rising concentrations of prostaglandin E2 (PGE2), while the decline in lung liquid volume is associated with increased production of cortisol and catecholamines, from late pregnancy to birth (Jain & Duddell 2006, Lagercrantz & Herlenius 2002). It is suggested that the fetal brain is protected from reduced oxygen and glucose supplies around the time of birth, by an increase in central oxytocin, beginning just before the onset of labour and peaking around 2 hours before birth (Brown & Grattan 2007). The fetal brain is exposed to elevated levels of oxytocin, which triggers a transient but significant switch of the GABA neurotransmitter, from excitatory to inhibitory. This reduces nutrient and oxygen requirements of the brain during transition to air-breathing and suckling (Khazipov et al 2008, Tyzio et al 2006). Current evidence suggests that the oxytocin is derived from both mother and fetus (Khazipov et al 2008).
The fetal adreno-placental ‘clock’
Duration of pregnancy is strongly associated with the rising profile of placental CRH in the maternal circulation (Smith 2007, Tyson et al 2009). CRH levels rise exponentially in maternal and fetal circulatory systems during the last 12 weeks of pregnancy, peak during labour, and fall precipitously following birth (Chan et al 1993, Goland et al 1986). In individual women, the exponential increase tends to mirror the duration of pregnancy: women who give birth prematurely have higher mid-pregnancy levels of CRH than those who give birth at term (McLean et al 1995, Smith 2007). The bioavailability of CRH is regulated by a circulating binding protein, which declines at the end of pregnancy, further increasing maternal and fetal tissue exposure to CRH (Grammatopoulos 2008) (Fig. 35.1).
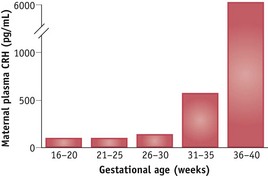
Figure 35.1 Mean plasma CRH concentrations in eight women followed sequentially during the second half of pregnancy.
(Reproduced with permission from Goland et al 1994:1289.)
During pregnancy and labour, placental CRH targets a number of maternal, placental and fetal organ systems. In the fetal compartment, CRH receptors have been identified in the pituitary gland, adrenal cortex, lungs, placenta and membranes (Grammatopoulos 2007, 2008). In the adrenals, placental CRH directly stimulates the fetal zone to produce DHEA-S and the definitive zone to produce cortisol, in a dose-dependent manner (Rehman et al 2007). The maternal pituitary–adrenal axis is also a target organ for CRH, while the myometrium is both a source and target for CRH and a related family of urocortin peptides (Goland et al 1994, Grammatopoulos 2007, Markovic et al 2007, Smith 2007) (see website).
Myometrial actions of placental CRH
As term approaches, oxytocin and inflammatory cytokines also stimulate expression of many variants of the CRH-R1 receptor with reduced signalling capacities (Grammatopoulos & Hillhouse 1999, Hillhouse & Grammatopoulos 2001). Recent work suggests that expression of CRH-R1 variants with reduced signalling capacity are only increased in the lower uterine segment with the onset of labour (Markovic et al 2007).
In contrast to CRH-R1, activation of CRH-R2 stimulates signalling pathways that enhance myometrial contractility. Recent experiments on gene profiles in different regions of the uterus have identified expression of fundal genes for CRH-R2 that increase significantly during labour (Grammatopoulos 2008, Stevens & Challis 1998). These findings indicate that dynamic changes in the balance of myometrial CRH-R1 receptor subtypes at term stimulate concomitant physiological changes in the fundus and lower uterine segment from late pregnancy to birth. While muscles in the upper segment generate coordinated forceful contractions, those in the lower segment have reduced stimulatory influences, which facilitates increased contractility of the fundus, elongation of the lower segment over the presenting part and progressive cervical dilation, as labour advances (Bukowski et al 2006).
CRH activity in placenta and membranes
The placenta and membranes also express two major CRH receptor subtypes: CRH-R1 and CRH-R2. In the placenta, the CRH-R1 subtype seems to increase expression of type 2 cyclo-oxygenase (COX-2), which stimulates biosynthesis of prostaglandin precursors and decreases expression of prostaglandin dehydrogenase (PGDH) – the key enzyme produced by the placenta and membranes that metabolizes active primary prostaglandins to an inactive form and inhibits production of progesterone (Amash et al 2009, Gao et al 2008, Grammatopoulos 2008) (see website).
Uterine oxytocin receptors
During pregnancy, myometrial receptors for oxytocin (OTR) increase from 27.6 fmol/mg DNA in the non-pregnant state to 171.6 fmol/mg DNA at mid-gestation, and 1391 fmol/mg DNA at term (Fuchs et al 1984). This represents a 50-fold increase within the uterus before the onset of labour (see website).
Maximum receptor concentrations have been found in early labour at term, 3583 fmol/mg DNA – significantly higher than before labour begins (Fuchs et al 1982). Concentrations of decidual receptors are relatively low in mid-pregnancy and reach maximal values following the onset of labour. Within the fetal membranes, increased OTR binding has been found between late pregnancy and labour, with highest increases in the amnion (Takemura et al 1994). Myometrial receptor concentrations are highest in the fundus and corpus, significantly lower in the lower segment, and lowest in the cervix, while decidual receptors are highest in sections surrounding the corpus and lowest around the lower segment (Blanks et al 2003).
During early labour, myometrial receptor concentrations are uniformly high in the upper segment and progressively lower in the isthmus and cervix, while those in the decidua are highest in the corpus, followed by the fundus and the isthmus (Fuchs et al 1984, Fuchs & Fuchs 1991, Hirst et al 1993) (see website).
There is an important role for gap junctions in coordinating cellular responsiveness to oxytocin. At term, higher concentrations of myometrial gap-junctions occur in the fundus compared to the lower segment, and the difference becomes increasingly pronounced during labour. This creates increasing fundal dominance during the course of labour and regulates progressive conductance of electrical activity, from fundus to cervix, to propagate multicellular synchronization of myometrial responsiveness to neuroendocrine, pulsatile and intrauterine oxytocin systems (Blanks et al 2003, Fuchs et al 1991, Kimura et al 1996, Russell et al 2003, Shmygol et al 2006).
During spontaneous labour, myometrial and decidual OTR concentrations decline significantly in advanced labour, particularly in the lower segment (Fuchs et al 1984). While findings in the lower segment are unreliable because of progressive incorporation of the cervix into the lower segment, available evidence suggests that oxytocin receptor mRNA significantly declines in the lower segment with increasing duration of labour. In spontaneous labour, the decline occurs gradually over 12–16 hours, but in oxytocin-induced and oxytocin-augmented labour, it is much steeper, especially when the infusion is constant rather than pulsatile (Phaneuf et al 2000, Robinson et al 2003, Willcourt et al 1994) (see website).
Nocturnal myometrial activation and cervical ripening
The uterus has a well-defined 24-hour rhythm of contractility and electrical and endocrine activation (Schlabritz-Loutsevitch et al 2003). In human pregnancy, increased contractile activity has been observed between 20:30 and 02:00 from 24 weeks’ gestation (Fuchs et al 1992, Germain et al 1993, Moore et al 1994, Sharkey et al 2009). Current research suggests the emergence of a nocturnal surge in rhythmic myometrial contractions is a key indication of uterine activation in preparation for the shift from pregnancy to labour. Nocturnal surges in oestradiol, melatonin and oxytocin occur from around 35–36 weeks’ gestation and these coincide with the 24-hour rhythm of spontaneous birth (Fuchs et al 1992, Schlabritz-Loutsevitch et al 2003, Tamura et al 2008). The nocturnal surge in oestriol, which originates almost exclusively from fetal adrenal DHEA-S, occurs from 35 weeks’ gestation; nocturnal plasma melatonin rises from 36 weeks’ gestation and nocturnal peaks in plasma concentrations of oxytocin occur from 37–39 weeks’ gestation (Fuchs et al 1991, 1992, Germain et al 1993, Moore et al 1994, Murphy Goodwin 1999, Schlabritz-Loutsevitch et al 2003, Tamura et al 2008). Oestradiol and melatonin increase gap junctions and oxytocin receptors, and melatonin also synergizes with oxytocin, increasing oxytocin-induced contractility in a dose-dependent manner (Sharkey et al 2009).
From approximately 36 weeks onwards, structural alterations become more apparent in cervical stroma and mucosal tissues, which alters its dimensions in relation to the lower uterine segment (House et al 2009). Within cervical connective tissue, alterations occur in the composition and concentration of the gel-like material called ground substance (proteoglycans) in which connective tissue cells and fibres are embedded. At the same time, an increase occurs in enzymes that degrade collagen. The concentration of ground substance relative to collagen is thought to reach a maximum during cervical softening prior to the onset of labour. This overall increase is characterized by the emergence of a higher proportion of molecules with a weaker affinity for collagen fibrils (see website).
Cervical and uterine muscles
During late pregnancy and the latent phase of labour, myometrial components of the cervix contract in characteristic short, high-frequency pressure increases, that are independent of the rest of the uterus, until the onset of established labour (Rudel & Pajntar 1999). These contractions may stimulate local connective tissue changes associated with cervical ripening (Olah et al 1993, Pajntar 1994). In primiparous women, softening of cervical tissue proceeds alongside effacement and is thought to occur in response to increased formation of gap junctions between adjacent cells, in the myometrium of the uterine cavity. Gap junctions are composed of symmetrical portions of plasma membrane from adjacent cells. These form intercellular channels for passage of ions and small molecules, facilitating rapid intracellular transmission of electrical impulses and chemical signals between cells. Gap junctions emerge in late pregnancy and undergo further increases in size and number during early labour. Formation and permeability of gap junctions are stimulated by oestrogens, prostaglandins and melatonin, and inhibited by progesterone, hCG and relaxin (Ambrus & Rao 1994, Burghardt et al 1993, Chow & Lye 1994, Sharkey et al 2009). Before labour begins, myometrial expression of gap junctions is much higher in the fundus than in the lower segment and this difference accelerates during the course of labour (Sparey et al 1999).
By facilitating the propagation of action potentials from cell to cell, gap junctions synchronize myometrial activity. Tension is transmitted from the myometrium by the outer layer of muscle that extends along the periphery of the supravaginal portion of the cervix (Pajntar 1994). This facilitates stretching of the lower uterine segment, which elongates as pressure is exerted by the fetus during descent into the pelvis. These combined forces seem to produce a differential rate of tissue uptake in the cervix and the adjacent lower segment of the uterus. Maximum uptake occurs at the lower peripheral end of the cervix, producing a gradual upward movement of soft cervical tissue that eventually merges with the lower segment (Gee & Olah 1993, Havelock et al 2005) (Fig. 35.2).
In a recent study on women following induction and augmentation of labour, a number of significant features were associated with the presence or absence of cervical contractions in response to myometrial activity. Cervical contractions predominantly occurred in women with lower measures of cervical effacement and dilatation and a longer latent phase, compared to those in whom cervical contractions were absent (Rudel & Pajmtar 1999). These indicate the importance of coordinated changes, from the fundus to the cervix, during the transition from pregnancy to labour (Havelock et al 2005, Olah et al 1993, Pajntar 1994, Rudel & Pajntar 1999).
Uterocervical changes and inflammation
Local pro-inflammatory changes accompany the remodelling and stretching of uterine muscle and cervical connective tissue during the latter part of pregnancy. The progressive release of inflammatory mediators like nuclear factor kappa B (NF-κB), cytokines and interleukins seems to gradually overwhelm the selective suppression of inflammatory and immune responses established from the beginning of pregnancy by progesterone, prolactin and cortisol (Gubbay et al 2002, Johnson et al 2008, Lindstrom & Bennett 2005, Pepe & Albrecht 1995, Rosen et al 1998, Shynlova et al 2009, Vaisanen-Tommiska et al 2003). Remodelling of the cervical connective tissue; stretching of the lower uterine segment and of the fetal membranes overlying the cervix, produce local alterations in the relative activity of mediators of inflammatory and anti-inflammatory reactions (Allport et al 2001, Bennett et al 2001, Moore et al 2006, Vaisanen-Tommiska et al 2003).
These include increased concentrations of a key cytokine, interleukin (IL)-8, in the cervix and lower uterine segment with cervical ripening; higher concentrations of enzymes that synthesize prostacyclin, PGE2 and PGF2α in the lower segment compared to the fundus, before and during labour; raised cervical production of cytokines and nitric oxide (NO) at term and during labour; increased expression of NF-κB and decreased expression of glucocorticoid receptors in cervical tissue from late pregnancy to birth; and downregulation of placental cortisol receptors (Allport et al 2001
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree