Robin Chard
Care of Postoperative Patients
Learning Outcomes
Safe and Effective Care Environment
Health Promotion and Maintenance
Physiological Integrity
6 Perform an ongoing head-to-toe assessment of the postoperative patient.
11 Evaluate surgical incisions and wounds for complications.

http://evolve.elsevier.com/Iggy/
Answer Key for NCLEX Examination Challenges and Decision-Making Challenges
Audio Clip: Stridor
Audio Glossary
Concept Map Creator
Key Points
Review Questions for the NCLEX® Examination
Completion of surgery and transfer of the patient to either the postanesthesia care unit (PACU), the same-day surgery (SDS) unit (ambulatory care unit), or the intensive care unit (ICU) mark the beginning of the postoperative period. Patients are discharged from these areas when they are stable and have met specific discharge criteria. Many patients can be discharged to home shortly after the surgery is completed. They are observed and monitored until discharge criteria are met and are then discharged. Some patients move from the specialized nursing care in the PACU to a hospital inpatient floor or ICU for additional nursing care.
The postoperative period continues after the patient’s condition is stabilized, as well as after the patient is discharged from the ambulatory surgery facility or hospital. The actual time spent away from home after surgery varies according to age, physical health, self-care ability, support systems, type and length of surgical procedure, anesthesia, any complications, and community resources. The measures recommended by the Surgical Care Improvement Project (SCIP) that were initiated during the preoperative period to prevent certain surgical complications are continued or re-evaluated during the postoperative period. (See Chapter 16 and Table 16-1 for an explanation of these measures.)
Overview
The purpose of a postanesthesia care unit (PACU) (recovery room) is the ongoing evaluation and stabilization of patients to anticipate, prevent, and manage complications after surgery. The PACU is usually located close to the surgical suite for ease of access and patient transfer. The unit is usually a large and open room to provide direct observation of all patients and easy access to supplies and emergency equipment. Adults are usually separated from children. The patient area may be divided into individual cubicles. So that each patient can be observed continuously, privacy curtains or screens are closed only during bedside procedures. Each cubicle has equipment to monitor and care for the patient, such as oxygen, suction equipment, cardiac monitors, pulse oximetry, airway equipment, and emergency drugs.
After the surgery is completed, the circulating nurse and the anesthesia provider accompany the patient to the PACU. When the patient is in critical condition, transfer may be directly from the operating room (OR) to the ICU. On arrival, The Joint Commission’s National Patient Safety Goals (NPSGs) require that the anesthesia provider and the circulating nurse give the PACU nurse a verbal “hand-off” report to communicate the patient’s condition and care needs.
A hand-off report that meets National Patient Safety Goal 2 requires effective communication between health care professionals. It is at least a two-way verbal interaction between the health care professional giving the report and the nurse receiving it. The language used by the person or persons giving the report is clear and cannot be interpreted in more than one way. The nurse receiving the report focuses on the report and is not distracted by the environment or other responsibilities. Standardizing the information reported helps prevent omission of critical patient-centered information and helps avoid irrelevant details (Association of periOperative Registered Nurses [AORN], 2010). The receiving nurse takes the time to restate (report back) the information to verify what was said and to make certain both the reporting person and the receiving nurse have the same understanding. The receiving nurse takes the time to ask questions and the reporting professional must respond to the questions until a common understanding is established. Chart 18-1 gives an example of critical information to include in a standard hand-off report.
The PACU nurse is skilled in the care of patients with multiple medical and surgical problems immediately after a surgical procedure. This area requires in-depth knowledge of anatomy and physiology, anesthetic agents, pharmacology, pain management, extubation, surgical procedures, and advanced cardiac life support (ACLS). The PACU nurse is skilled in assessment and can make quick decisions if emergencies or complications occur. The patient is monitored closely. The anesthesia provider and surgeon are consulted as needed.
Patient-Centered Collaborative Care
Assessment
History
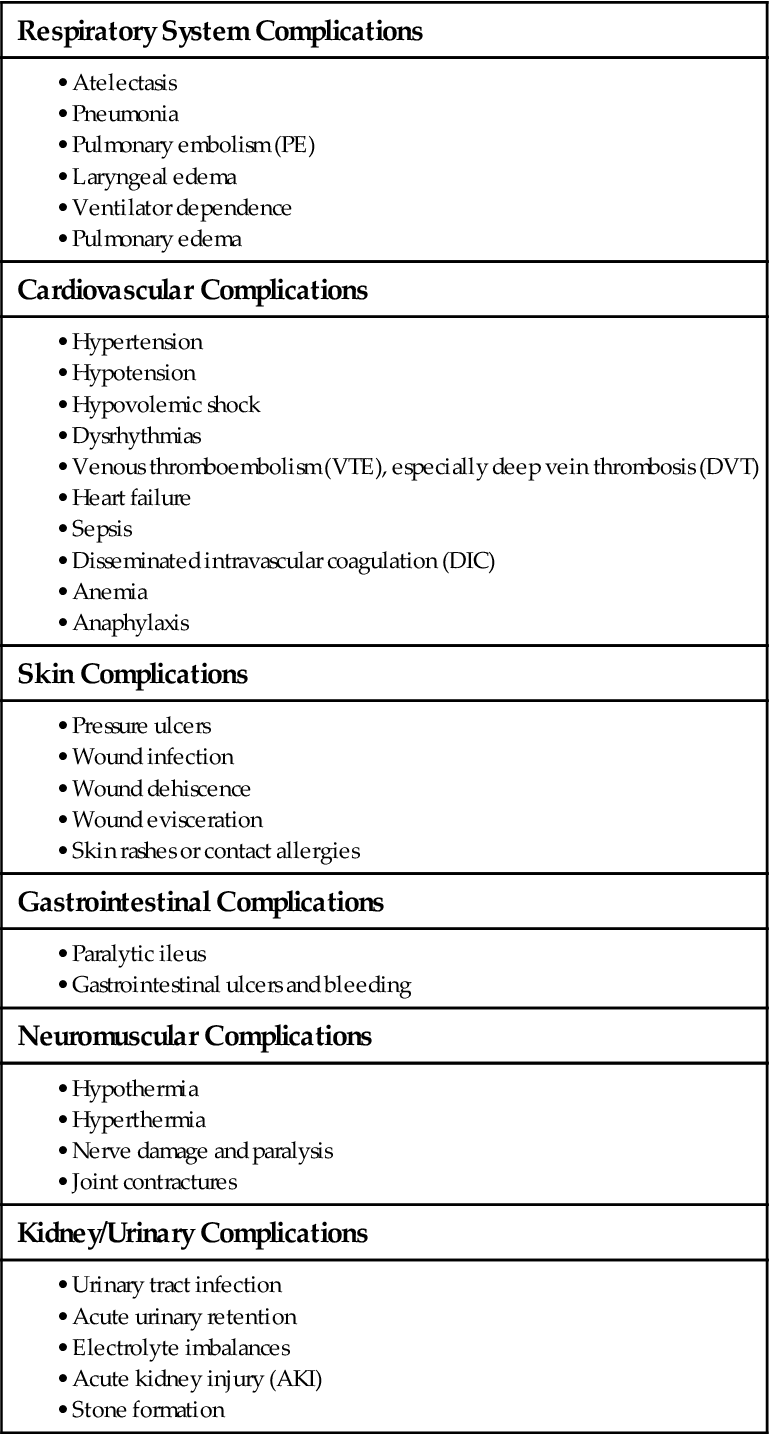
Use the surgical team’s report to plan the care for an individual patient. After receiving the report and assessing the patient, review the medical record for information about the patient’s history, presurgical physical condition, and emotional status. If the patient remains as an inpatient, the surgical and anesthesia information is incorporated into the postoperative plan of care. Chapter 16 identifies situations that increase a patient’s risk for the potential complications listed in Table 18-1.
TABLE 18-1
GENERAL POTENTIAL COMPLICATIONS OF SURGERY
| Respiratory System Complications |
| Cardiovascular Complications |
| Skin Complications |
| Gastrointestinal Complications |
| Neuromuscular Complications |
| Kidney/Urinary Complications |
Physical Assessment/Clinical Manifestations
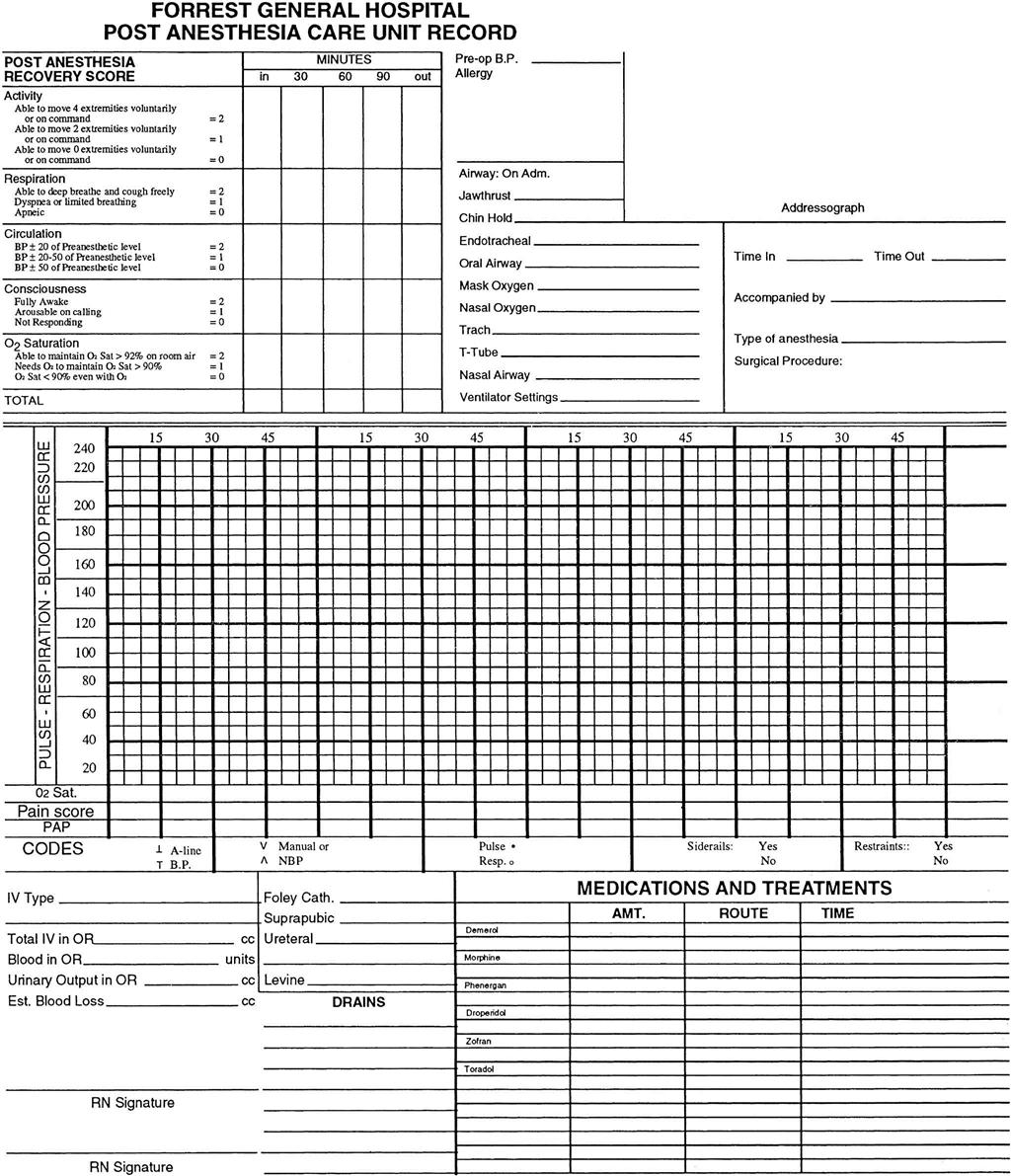
Assess the patient, and record data on a PACU flow chart record (Fig. 18-1). Assessment data include level of consciousness, temperature, pulse, respiration, oxygen saturation, and blood pressure. Examine the surgical area for bleeding. Monitor vital signs as often as your facility’s policy states, the patient’s condition warrants, and the surgeon prescribes. Once the patient is discharged from the PACU, vital signs are often measured every 15 minutes for four times, every 30 minutes for four times, every hour for four times, and then every 4 hours for 24 to 48 hours if the patient’s condition is stable. Thereafter, if the patient is admitted, vital signs are assessed according to the facility’s policy, the patient’s condition, and the nurse’s judgment.
The health care team determines the patient’s readiness for discharge from the PACU by the presence of a recovery score rating of 9 to 10 on the recovery scale (see Fig. 18-1). Other criteria for discharge (e.g., stable vital signs; normal body temperature; no overt bleeding; return of gag, cough, and swallow reflexes; the ability to take liquids; and adequate urine output) may be specific to the facility. After you determine that all criteria have been met, the patient is discharged by the anesthesia provider to the hospital unit or to home. If an anesthesia provider has not been involved, which may be the case with local anesthesia or moderate sedation, the surgeon or nurse discharges the patient once the discharge criteria have been met.
Assessment continues from the PACU to the intensive care or medical-surgical nursing unit. If the patient is to be discharged from the PACU to home, assessment is continued by home care nurses or by the patient or family members after health teaching. When the patient is transferred to an inpatient unit, complete an initial assessment on arrival (Chart 18-2).
During the postoperative period, all patients remain at risk for pneumonia, shock, cardiac arrest, respiratory arrest, venous thromboembolism (VTE), and GI bleeding. These serious complications can be prevented or the consequences reduced with coordinated care. Nursing observations and interventions are part of critical rescue management for patient safety and quality care, as defined by The Joint Commission’s NPSGs, to reduce the risk for an adverse patient outcome after surgery.
Respiratory System.
When the patient is admitted to the PACU, immediately assess for a patent airway and adequate gas exchange. Although some patients may be awake and able to speak, talking is not a good indicator of adequate gas exchange. An artificial airway, such as an endotracheal tube (ET), a nasal trumpet, or an oral airway, may be in place. If the patient is receiving oxygen, document the type of delivery device and the concentration or liter flow of the oxygen. Continuously monitor pulse oximetry for oxygen saturation (SpO2) while the patient is in the PACU. The SpO2 should be above 95% (or at the patient’s presurgery baseline).
Assess the rate, pattern, and depth of breathing to determine adequacy of gas exchange. A respiratory rate of less than 10 breaths per minute may indicate anesthetic- or opioid analgesic–induced respiratory depression. Rapid, shallow respirations may signal shock, cardiac problems, increased metabolic rate, or pain.
Listen to the lungs over all lung fields to assess breath sounds. Also check symmetry of breath sounds and chest wall movement. If, for example, the patient has an ET tube, it could move down into the right mainstem bronchus and prevent left lung expansion. In this case, lung sounds on the left are absent or decreased and only the right chest wall rises and falls with breathing.
Perform ongoing inspection of the chest wall for accessory muscle use, sternal retraction, and diaphragmatic breathing. These signs could indicate an excessive anesthetic effect, airway obstruction, or paralysis, which could result in hypoxia. Listen for snoring and stridor (a high-pitched crowing sound). Snoring and stridor occur with airway obstruction resulting from tracheal or laryngeal spasm or edema, mucus in the airway, or blockage of the airway from edema or tongue relaxation. When neuromuscular blocking agents are retained, the patient has muscle weakness, which could impair gas exchange. Indicators of muscle weakness include the inability to maintain a head lift, weak hand grasps, and an abdominal breathing pattern.
If the patient returns to an inpatient unit, complete an initial assessment on arrival (see Chart 18-2) and then continue to assess for respiratory depression or hypoxemia. Listen to the lungs to check for effective expansion and for abnormal breath sounds. Check the lungs at least every 4 hours during the first 24 hours after surgery and then every 8 hours, or more often, as indicated. Older patients, smokers, and patients with a history of lung disease are at greater risk for respiratory complications after surgery and need more frequent assessment (Sullivan, 2011). Obese patients are also at high risk for respiratory complications.
Cardiovascular System.
Vital signs and heart sounds are assessed on admission to the PACU and then at least every 15 minutes until the patient’s condition is stable. Automated blood pressure cuffs and cardiac monitoring assist in continuous assessment.
Review vital signs after surgery for trends, and compare them with those taken before surgery. Report blood pressure changes that are 25% higher or lower than values obtained before surgery (or a 15- to 20-point difference, systolic or diastolic) to the anesthesia provider or the surgeon. Decreased blood pressure and pulse pressure and abnormal heart sounds indicate possible cardiac depression, fluid volume deficit, shock, hemorrhage, or the effects of drugs (see Chapters 13 and 39). Bradycardia could indicate an anesthesia effect or hypothermia. Older patients are at risk for hypothermia because of age-related changes in the hypothalamus (the temperature regulation center), low levels of body fat, and coolness of the OR suite (Touhy & Jett, 2010). An increased pulse rate could indicate hemorrhage, shock, or pain.
Cardiac monitoring is maintained until the patient is discharged from the PACU. For patients at risk for dysrhythmias, monitoring may continue either on telemetry units or on general medical-surgical units. In assessing the vital signs of a patient who is not being monitored continuously, compare the rate, rhythm, and quality of the apical pulse with the rate, rhythm, and quality of a peripheral pulse, such as the radial pulse. A pulse deficit (a difference between the apical and peripheral pulses) could indicate a dysrhythmia.
Peripheral vascular assessment needs to be performed because anesthesia and positioning during surgery (e.g., the lithotomy position for genitourinary procedures) may impair the peripheral circulation and contribute to venous thromboembolism (VTE), especially deep vein thrombosis (DVT) (Owens, 2008). Compare distal pulses on both feet for the quality of pulsation, observe the color and temperature of extremities, evaluate sensation, and determine the speed of capillary refill. Palpable pedal pulses indicate adequate circulation and perfusion of the legs.
In adherence to The Joint Commission’s Surgical Care Improvement Project’s (SCIP) core measures for prevention of VTE, continue the prophylactic measures initiated before surgery. Although these measures vary in type (e.g., drug therapy with anticoagulants or antiplatelet drugs, sequential compression devices, antiembolic stockings or elastic wraps, early ambulation) depending on the patient’s specific risk factors and the type and extent of surgery, any preventive strategies started before surgery are usually needed for at least the first 24 hours after surgery. Reassessment of the patient’s risk for VTE and the effectiveness of the preventive strategies is performed daily. Assess the feet and legs for redness, pain, warmth, and swelling, which may occur with DVT. Foot and leg assessment may be performed once during a nursing shift, once daily, or once per visit, depending on the patient’s risk for complications and the facility’s or agency’s policy. (See Chapters 16 and 38 for more information on VTE.)
Neurologic System.
Cerebral functioning and the level of consciousness or awareness must be assessed in all patients who have received general anesthesia (Table 18-2) or any type of sedation. Observe for lethargy, restlessness, or irritability, and test coherence and orientation. Determine awareness by observing responses to calling the patient’s name, touching the patient, and giving simple commands such as “Open your eyes” and “Take a deep breath.” Eye opening in response to a command indicates wakefulness or arousability but not necessarily awareness. Determine the degree of orientation to person, place, and time by asking the conscious patient to answer questions such as “What is your name?” (person), “Where are you?” (place), and “What day is it?” (time).
TABLE 18-2
IMMEDIATE POSTOPERATIVE NEUROLOGIC ASSESSMENT: RETURN TO PREOPERATIVE LEVEL
| Order of Return to Consciousness After General Anesthesia |
| Order of Return of Motor and Sensory Functioning After Local or Regional Anesthesia |
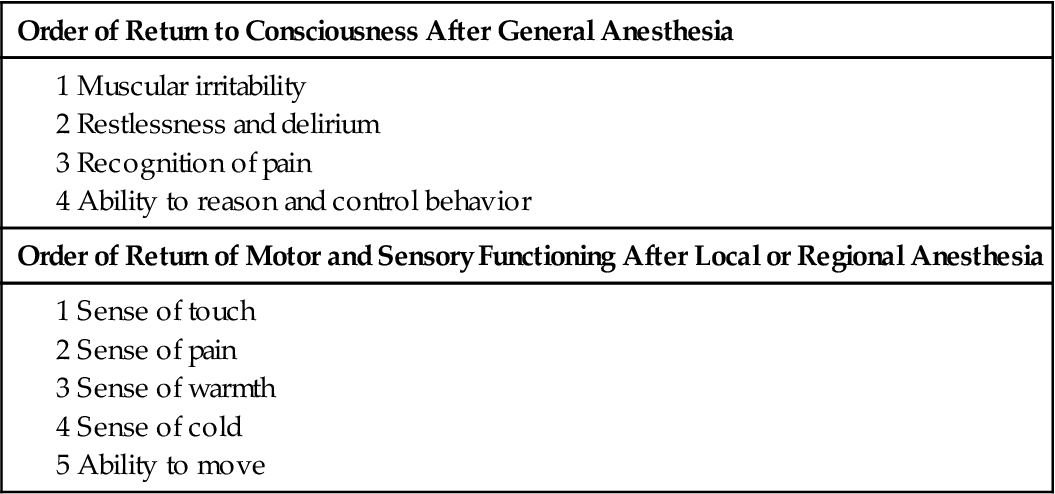
Compare the patient’s baseline neurologic status (obtained before surgery) with the findings after surgery. Patients who had altered cerebral functioning before surgery because of another condition usually continue to have that alteration after surgery. After the patient is alert (and all other criteria have been met), he or she is discharged from the PACU. On the medical-surgical nursing unit, assess the level of consciousness every 4 to 8 hours or as indicated by the patient’s condition and the facility’s policy.
Motor function and sensory function are assessed for all patients who received general or regional anesthesia. General anesthesia depresses all voluntary motor function. Regional anesthesia alters the motor and sensory function of only part of the body. (See Chapter 17 for more information on anesthesia.) Motor and sensory assessment are very important after epidural or spinal anesthesia. Evaluate motor function by asking the patient to move each extremity. The patient who had epidural or spinal anesthesia remains in the PACU until sensory function (feeling) and voluntary motor movement of the legs have returned (see Table 18-2). Also assess the strength of each limb, and compare the results on both sides.
Test for the return of sympathetic nervous system tone by gradually elevating the patient’s head and monitoring for hypotension. Begin this evaluation after the patient’s sensation has returned to at least the spinal dermatome level of T10. (See Chapter 43 for further neurologic assessment.) After the patient is transferred to the nursing unit, continue neurologic assessment as indicated.
Fluid, Electrolyte, and Acid-Base Balance.
Fasting before and during surgery, the loss of fluid during the procedure, and the type and amount of blood or fluid given affect the patient’s fluid and electrolyte balance after surgery. Fluid volume deficit or fluid volume overload may occur after surgery. Sodium, potassium, chloride, and calcium imbalances also may result, as may changes in other electrolyte levels. Fluid and electrolyte imbalances occur more often in older or debilitated patients and in those with health problems such as diabetes mellitus, Crohn’s disease, or heart failure.
Intake and output measurement is part of the operative record and is reported by the circulating nurse to the PACU nurse. Record any intake or output, including IV fluid intake, vomitus, urine, wound drainage, and nasogastric (NG) tube drainage. You must know the total intake and output from both the OR and the PACU to assess fluid balance accurately and to complete the 24-hour intake and output record.
Hydration status is assessed in the PACU and the medical-surgical unit. To determine hydration status, inspect the color and moisture of mucous membranes; the turgor, texture, and “tenting” of the skin (test over the sternum or forehead of an older patient); the amount of drainage on dressings; and the presence of axillary sweat. Measure and compare total output (e.g., NG tube drainage, urine output, wound drainage) with total intake to identify a possible fluid imbalance. Consider insensible fluid loss, such as sweat, when reviewing total output. Continue to assess intake and output as long as the patient is at risk for fluid imbalances. Some facilities require intake and output to be measured if the patient receives IV fluids or has a catheter, drains, or an NG tube. In addition, patients who have heart disease or kidney disease may need a longer period of intake and output measurement.
IV fluids are closely monitored to promote fluid and electrolyte balance. Isotonic solutions such as lactated Ringer’s (LR), 0.9% sodium chloride (normal saline), and 5% dextrose with lactated Ringer’s (D5/LR) are used for IV fluid replacement in the PACU. After the patient returns to the medical-surgical unit, the type and rate of IV infusions are based on need. A typical IV solution for the patient being admitted to the nursing unit is 5% dextrose with 0.45% normal saline (D5 0.45% NS). (See Chapters 13 and 15 for further discussion of IV fluids, electrolyte balance, and hydration assessment.)
Acid-base balance is affected by the patient’s respiratory status before and during surgery; metabolic changes during surgery; and losses of acids or bases in drainage. For example, NG tube drainage or vomitus causes a loss of hydrochloric acid and leads to metabolic alkalosis. Examine arterial blood gas (ABG) values and other laboratory values. (See Chapter 14 for more detailed information on acid-base imbalances.)
Kidney/Urinary System.
Control of urination may return immediately after surgery or may not return for hours after general or regional anesthesia. The effects of preoperative drugs (especially atropine), anesthetic agents, or manipulation during surgery can cause urine retention. Assess for urine retention by inspection, palpation, and percussion of the lower abdomen for bladder distention or by the use of a bladder scanner (see Chapter 68). Assessment may be difficult to perform after lower abdominal surgery. Urine retention is common early after surgery and requires intervention, such as intermittent (straight) catheterization, to empty the bladder.
When the patient has an indwelling urinary (Foley) catheter, assess the urine for color, clarity, and amount. If the patient is voiding, assess the frequency, amount per void, and any symptoms. Urine output should be close to the total intake for a 24-hour period. Consider other sources of output, such as sweat, vomitus, or diarrhea stools. Report a urine output of less than 30 mL/hr (240 mL per 8-hour nursing shift) to the physician. Decreased urine output may indicate hypovolemia or renal complications. (See Chapter 68 for kidney/urinary assessment.)
Gastrointestinal System.
Postoperative nausea and vomiting (PONV) are among the most common reactions after surgery. About 30% of patients who receive general anesthesia have some form of GI upset within the first 24 hours after surgery; however, some patients are more at risk than others (Bopp et al., 2010; Fetzer, 2008). Patients with a history of motion sickness are more likely to develop nausea and vomiting after surgery. Obese patients may be at risk because many anesthetics are retained by fat cells and remain in the body longer. Abdominal surgery and the use of opioid analgesics reduce intestinal peristalsis after surgery. These problems increase the risk for prolonged nausea and vomiting after surgery. Preventive drug therapy is effective in reducing the incidence. Drugs often used are ondansetron (Zofran), a serotonin antagonist, and meclizine (Antivert, Dramamine), a sedating H1 histamine antagonist (Bopp et al., 2010).
PONV can stress and irritate abdominal and GI wounds, increase intracranial pressure in patients who had head and neck surgery, elevate intraocular pressure in patients who had eye surgery, and increase the risk for aspiration. Assess the patient continuously for PONV. Often patients have nausea as the head of the bed is raised early after surgery. This symptom may occur with or without dizziness. You can help reduce this distressing symptom by having the patient in a side-lying position before raising the head slowly.
Intestinal peristalsis may be delayed because of prolonged anesthesia time, the amount of bowel handling during surgery, and opioid analgesic use. In the PACU and later on the medical-surgical unit, assess for the return of peristalsis. Patients who are recovering from abdominal surgery often have decreased or no peristalsis for at least 24 hours. This problem may persist for several days for those who have GI surgery.
Listen for bowel sounds in all four abdominal quadrants and at the umbilicus. If NG suction is being used, turn off the suction before listening to prevent mistaking the sound of the suction for bowel sounds. The presence of active bowel sounds usually indicates return of peristalsis; however, the absence of bowel sounds does not confirm a lack of peristalsis. The best indicator of intestinal activity is the passage of flatus or stool. Abdominal cramping along with distention denotes trapped, nonmoving gas—not peristalsis.
Decreased peristalsis occurs in patients who have a paralytic ileus. The abdominal wall is distended, and there is no movement of the intestinal wall. Assess for the manifestations of paralytic ileus (few or absent bowel sounds, a distended abdomen, abdominal discomfort, vomiting, no passage of flatus or stool).
A nasogastric (NG) tube may be inserted during surgery to decompress and drain the stomach, to promote GI rest, and to allow the lower GI tract to heal. It may also be used to monitor any gastric bleeding and to prevent intestinal obstruction. One of the most common tubes used is the Salem sump. The Salem sump is a double-lumen tube with an air vent to keep the tube from grabbing the gastric mucosa. This feature allows easy drainage of the stomach and prevents mucosal damage. Less commonly used is the Levin tube, which is a single-lumen tube with no air vent. To promote drainage, suction (usually low) is applied to the NG tube. Suction is either continuous (recommended for the Salem sump) or intermittent.
Record the color, consistency, and amount of the NG drainage every 8 hours (Table 18-3). In some instances, an occult blood test (Gastroccult) may be performed. Normal NG drainage fluid is greenish yellow. Red drainage fluid indicates active bleeding, and brown liquid or drainage with a “coffee-ground” appearance indicates old bleeding. Assess that the NG tube is securely taped to the nose, and note any skin irritation.
TABLE 18-3
CALCULATING NASOGASTRIC TUBE DRAINAGE
| Formula |
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
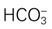 21 mEq/L, Pa
21 mEq/L, Pa