Rachel L. Palmieri
Care of Patients with Problems of the Peripheral Nervous System
Learning Outcomes
Safe and Effective Care Environment
Health Promotion and Maintenance
Psychosocial Integrity
Physiological Integrity
5 Describe how to perform focused neurologic assessments for patients with PNS disorders.
6 Compare and contrast the pathophysiology and etiology of GBS and MG.
7 Prioritize nursing care for the patient with GBS or MG.
8 Differentiate between a myasthenic crisis and a cholinergic crisis.
9 Identify specific nursing actions regarding drug administration for the patient with MG.
10 Assess patients having a thymectomy for postoperative complications.
11 Plan and implement postoperative care for the patient undergoing peripheral nerve repair.
12 Identify risk factors for restless legs syndrome.
13 Compare trigeminal neuralgia and facial paralysis assessment findings.
15 Identify the priority for care for patients with trigeminal neuralgia.

http://evolve.elsevier.com/Iggy/
Animation: Guillain-Barré Syndrome
Answer Key for NCLEX Examination Challenges and Decision-Making Challenges
Audio Glossary
Concept Map Creator
Key Points
Review Questions for the NCLEX® Examination
The peripheral nervous system (PNS) is composed of the spinal nerves, cranial nerves, and part of the autonomic nervous system. Its function is to provide communication from the brain and spinal cord to other parts of the body. Neuropathy or peripheral neuropathy (PN) is a global word that refers to any disease, disorder, or damage to the PNS. These health problems may be acute, such as Guillain-Barré syndrome, or chronic, such as diabetic neuropathy. Typical clinical manifestations of neuropathy include pain, muscle cramps, and muscle weakness.
Acquired neuropathies are grouped into three broad categories: inflammatory, traumatic, or systemic. Inflammatory neuropathies such as Guillain-Barré syndrome can affect the entire peripheral nervous system. Trigeminal neuralgia is a type of neuropathy caused by trauma or damage to a single nerve—the trigeminal nerve. Examples of systemic neuropathy are diabetic PN and chemotherapy-induced PN. Neuropathies that are part of or caused by systemic diseases or treatments are discussed elsewhere in this text. All of these PNS health problems cause impairments in the human needs of mobility, sensation, or both.
Guillain-Barré Syndrome
Pathophysiology
Guillain-Barré syndrome (GBS) is an acute inflammatory demyelinating polyneuropathy (AIDP) that affects the peripheral nervous system, causing motor weakness and sensory abnormalities. It is an uncommon disorder, affecting both genders equally and peaking after age 55 years (Simmons, 2010). Euro-Americans have the disease more often than other racial or ethnic groups.
GBS may be referred to by a variety of other names, such as acute idiopathic polyneuritis and polyradiculoneuropathy. As a result of demyelination (destruction of the myelin sheath) of the peripheral nerves, progressive motor weakness and sensory abnormalities occur. Symptoms typically begin in the legs and spread to the arms and upper body. This is referred to as an ascending paralysis. Paralysis can increase in intensity until the muscles cannot be used at all and the patient is almost totally immobile. As a result, some patients require mechanical ventilation because of weak or paralyzed respiratory muscles. Healing occurs in reverse; the neurons affected last are the first to recover.
GBS is the result of a variety of related immune-mediated pathologic processes. The immune system starts to destroy the myelin sheath that surrounds the axons of the peripheral nerves or attacks the axons themselves. Segmental demyelination (the destruction of myelin between the nodes of Ranvier) is the major pathologic finding in GBS. This destruction affects the transmission of impulses from node to node by slowing it down. Also, the brain receives fewer sensory signals, affecting the patient’s ability to feel textures, heat, and pain. On the other hand, the brain may receive altered signals that cause the tingling or “crawling-skin” sensation many patients report.
On microscopic examination, groups of lymphocytes are seen at the points of myelin breakdown, yet the axons usually remain intact. In some instances, secondary damage to the cell body, the neurilemma, or the axon occurs. This can delay recovery or result in permanent neurologic deficits.
Three stages make up the acute course of GBS:
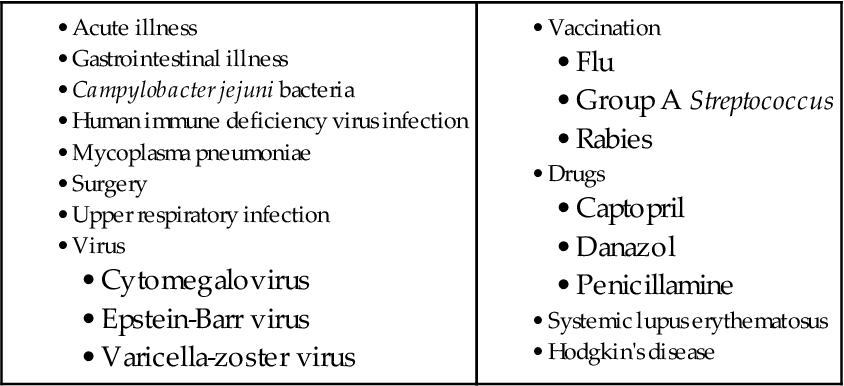
The exact cause of GBS remains unclear. The patient often relates a history of acute illness, trauma, surgery, or immunization 1 to 3 weeks before the onset of neurologic manifestations. Other risk factors include an upper respiratory tract infection or GI illness and positive antibodies to cytomegalovirus or Epstein-Barr virus (EBV). It is believed that the precipitating event or illness causes a limited malfunction of the immune system, which sensitizes the T-cells to the patient’s myelin. In response to several antigens, some patients apparently form a demyelinating antibody that has a direct toxic effect on nerves or attracts a cellular immune response. This ultimately destroys the myelin. Other factors associated with the development of GBS are listed in Table 46-1.
Patient-Centered Collaborative Care
Assessment
Obtain a complete health history. Ask the patient to describe GBS symptoms in chronologic order, if possible. Question about the presence of pain, which is common if the patient has paresthesias (unpleasant sensations such as burning, stinging, and prickly feeling).
Manifestations of GBS depend on the degree of weakness and the progression of symptoms. Although features vary, most people report a sudden onset of muscle weakness and pain (Chart 46-1). Typically, the disease does not affect level of consciousness, cerebral function, or pupillary constriction or dilation. The clinical variations of GBS reflect the areas of earliest or most severe involvement (Table 46-2).
TABLE 46-2
CLINICAL VARIANTS OF GUILLAIN-BARRÉ SYNDROME

With any of the variants, cranial nerve involvement most often affects the facial nerve (cranial nerve VII). Assess the patient’s ability to smile, frown, whistle, or drink from a straw. In addition to monitoring the functions of cranial nerve VII, assess the patient for dysphagia (difficulty swallowing). Less frequently affected cranial nerves include the glossopharyngeal (IX), vagus (X), accessory (XI), and hypoglossal (XII) nerves. The patient’s inability to cough, gag, or swallow results from the involvement of cranial nerves IX and X. Monitor the patient closely for varying blood pressure (hypertensive and hypotensive episodes or orthostatic hypotension), bradycardia, heart block, and, possibly, asystole. These symptoms are part of autonomic dysfunction, which is linked to vagus nerve (X) deficit. Assess cranial nerve XI (accessory) by asking the patient to shrug the shoulders. Hypoglossal nerve (XII) deficit is evidenced by an inability to stick the tongue out straight.
In addition to determining the usual roles and responsibilities, occupation, motivation, and available support systems, assess the patient’s ability to cope with this devastating illness and the accompanying fear and anxiety. In general, GBS is self-limiting and the paralysis is temporary. It is not unusual for the patient to have depression throughout the recovery period, however, due to a feeling of powerlessness.
Although no single clinical or laboratory finding confirms the diagnosis of GBS, the health care provider may perform a lumbar puncture (LP) to evaluate cerebrospinal fluid (CSF). An increase in CSF protein level often occurs due to the release of plasma proteins from inflammation, degeneration, and damage to nerve roots. However, high protein levels may not occur until after 1 to 2 weeks of illness, reaching a peak in 4 to 6 weeks. The CSF lymphocyte count is normal.
Peripheral blood tests may show a moderate leukocytosis early in the illness. The number of leukocytes rapidly returns to normal if there are no complications or concurrent illness.
Electrophysiologic studies (EPSs) demonstrate demyelinating neuropathy. The degree of abnormality found on testing does not always correlate with clinical severity. Within 3 weeks of symptoms, nerve conduction velocities are depressed. In some cases, denervated potentials (fibrillations) develop later in the illness. Electromyographic (EMG) findings, which reflect peripheral nerve function, are normal early in the illness. Electrophysiologic changes appear only after denervation of muscle has been present for 4 weeks or longer. Nerve conduction velocity (NCV) testing is performed with the EMG. Nerve damage or disease may still exist despite normal NCV results. These tests are described in Chapter 43.
A magnetic resonance imaging (MRI) or computed tomography (CT) scan may be requested to rule out other causes of motor weakness. Respiratory function is often compromised in patients with GBS. Therefore vital capacity may be decreased and arterial blood gas (ABG) values may be abnormal (decreased partial pressure of arterial oxygen [PaO2], increased partial pressure of arterial carbon dioxide [PaCO2], or increased pH).
Interventions
Managing Drug Therapy and Plasmapheresis
The health care provider follows the most recent best practice guidelines from the American Academy of Neurology (2011) for the treatment of GBS. These include:
Cerebrospinal fluid filtration (CSFF), also called liquorpheresis, has been a controversial new treatment option and lacking strong evidence for successfully managing Guillain-Barré syndrome. This procedure involves filtering out immunoglobulins and inflammatory mediators, such as cytokines.
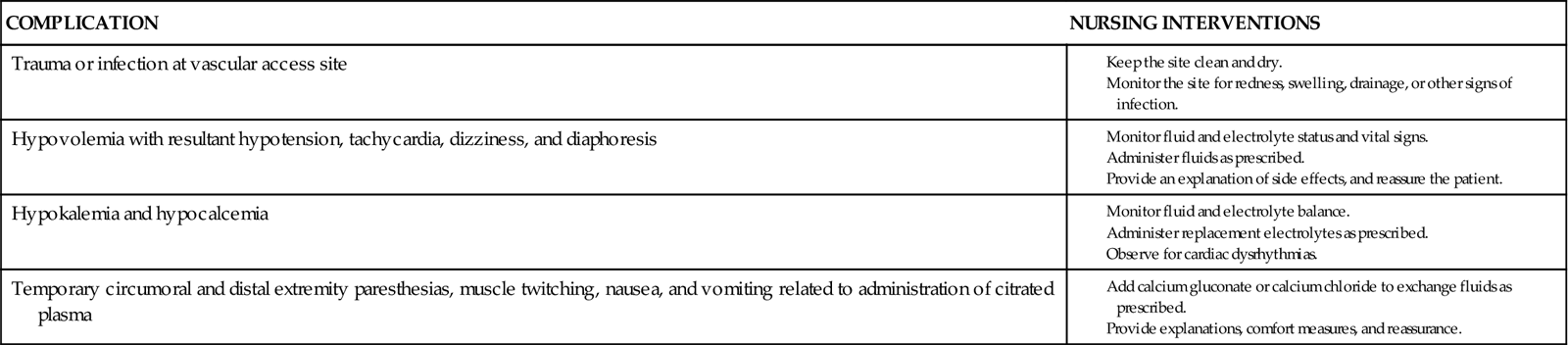
Plasmapheresis removes the circulating antibodies thought to be responsible for the disease. In this procedure, plasma is selectively separated from whole blood. The blood cells are returned to the patient without the plasma. Plasma usually replaces itself, or the patient is transfused with colloidal substitute such as albumin. Fresh frozen plasma is generally not used because of the associated risk for infection and allergic pulmonary edema. Plasmapheresis should be done within several days after the onset of the illness, although some patients benefit up to 30 days after the onset of symptoms. The patient usually receives three to four treatments, 1 to 2 days apart. Some patients may require a second round of treatment if they deteriorate after the first plasmapheresis.
Nursing interventions for the patient undergoing plasmapheresis include providing information and reassurance, weighing the patient before and after the procedure, and properly caring for the shunt, if used.
IVIG has been shown to be as effective as plasmapheresis. It is safer and immediately available. Side effects of immunoglobulin therapy range from minor annoyances (e.g., chills, mild fever, myalgia, headache) to major complications (e.g., anaphylaxis, aseptic meningitis, retinal necrosis, acute renal failure). A serum IgA is drawn before administration of the medication. Infuse IVIG slowly when it is started. Observe for side and adverse effects, and report their occurrence to the health care provider. The rate of administration can be increased based on the patient’s tolerance and on agency protocol.
Managing the Airway and Monitoring Respiratory Status
The priority nursing intervention of airway management is to promote airway patency and gas exchange. Elevate the head of the bed to at least 45 degrees or higher as determined by the patient’s response and level of dyspnea. The need for suctioning the patient is based on assessment data. During suctioning, the patient is at risk for vagal nerve stimulation that could lead to bradycardia and cardiac arrest. Monitor the color, consistency, and amount of secretions obtained. Chest physiotherapy, often performed by the respiratory therapist (RT), and frequent position changes are combined with breathing exercises (coughing and deep breathing) to prevent pneumonia and atelectasis. Encourage the patient to use the incentive spirometer to expand the lungs every few hours. Oxygen may be administered by nasal cannula at a flow rate prescribed by the health care provider.
Monitor ABG values or end-tidal carbon dioxide frequently for acid-base abnormalities; pulse oximetry reveals decreasing oxygen saturation. A decrease in vital capacity to less than 15 to 20 mL/kg (or less than two thirds of the patient’s normal) and the inability to clear secretions may be indications for elective intubation.
Managing Cardiac Dysfunction
Both the sympathetic and parasympathetic systems may be affected. Place the patient on a cardiac monitor because of the risk for dysrhythmias. Monitor vital signs closely. Hypertension is treated with a beta blocker or nitroprusside (Nitropress). Hypotension is treated with IV fluids and placing the patient in a supine position unless he or she is in extreme respiratory distress. Atropine may be prescribed to treat bradycardia.
Improving Mobility and Preventing Complications of Immobility
Collaborate with the patient, family, physical and occupational therapists (PT/OT), speech-language pathologist (SLP), and dietitian to develop interventions that prevent complications of immobility and to address deficits in self-care. Assess the patient’s motor (muscle) function every 2 to 4 hours as part of the neurologic assessment. The interventions prescribed for mobility and self-management and to prevent complications depend on the degree of motor deficit. The PT and OT provide assistive devices and instructions for their use.
To ensure safety, assist the patient with ambulation, transfers from bed to chair, position changes, and maintenance of proper body alignment until he or she is able to perform these activities independently. Encourage maximum independence. Perform active or passive range-of-motion (ROM) exercises every 2 to 4 hours, or delegate this activity to unlicensed assistive personnel (UAP) with supervision. Teach family members these techniques. (See Chapter 8 for detailed discussion of ways to improve physical mobility and self-care, as well as ways to prevent immobility consequences.) Monitor the patient’s responses, including fatigue level. Provide adequate rest periods between activities and therapy sessions.
Decreased gastric motility, dysphagia, and depression can cause malnutrition. Collaborate with the dietitian to develop an individualized plan. The patient may require little assistance with feeding or may be totally dependent. If he or she cannot safely swallow food or liquids, enteral nutrition via feeding tube is prescribed. Weigh the patient three times a week, and monitor serum prealbumin each week.
Malnutrition places patients at risk for pressure ulcers, especially when they are immobile. Therefore pay special attention to skin care, including interventions to prevent skin breakdown. Instruct UAP to turn the patient a minimum of every 2 hours. Assess the skin for any areas of redness that may lead to pressure ulcers. If the bed does not have a pressure-reducing mattress, use a mattress overlay to help prevent skin breakdown. Document changes in the patient’s skin condition every 8 to 12 hours while in the acute care setting and at least daily in a transitional care or rehabilitation setting. If an ulcer develops, implement aggressive interventions to manage the wound. Chapters 8 and 27 discuss pressure ulcer care in detail.
Because pulmonary emboli and deep vein thromboses are common complications of immobility, the health care provider may prescribe prophylactic anticoagulant therapy, such as subcutaneous heparin or Lovenox. Antiembolism stockings and sequential compression stockings may be used to promote venous return. Be sure that stockings are removed at least once every 24 hours for 15 to 30 minutes. Other prevention measures are determined by agency policy or health care provider preference. Chapter 8 describes additional interventions to prevent complications of immobility and promote self-care.
Managing Pain
Assess the severity and nature of the patient’s pain, which is often worse at night. The patient may have paresthesia or hyperesthesia (extreme sensitivity to touch), deep muscle aches, and muscle stiffness. The typical pain experienced is often not relieved by drugs other than opiates, which can be administered via a patient-controlled analgesia (PCA) pump or continuous IV drip. Other drugs that are given include gabapentin (Neurontin) or tricyclic antidepressants.
Other pain control measures include frequent repositioning, massage, ice, heat, relaxation techniques, guided imagery, hypnosis, and distractions (e.g., music, visitors). Chapter 5 discusses these modalities and other pain relief measures in detail.
Promoting Communication
The patient may have difficulty communicating because the muscles required for the production of speech are weak, or he or she may be mechanically ventilated because the respiratory muscles are paralyzed. In either case, collaborate with the speech-language pathologist to develop a communication system. A simple technique involves eye blinking or moving a finger to indicate “yes” and “no.” A communication board or flash cards can be used with the letters of the alphabet or a list of common requests, such as the need to be repositioned or the need for pain medication. Computer or personal digital assistant (PDA) technology may also be used, depending on muscle function. Both the staff and the visitors must know how the patient’s communication system operates.
Providing Psychosocial Support
Teach the patient and family about the illness, and explain all diagnostic tests and treatments. Assess the patient and family for verbal and nonverbal behaviors that indicate powerlessness, anxiety, fear, and isolation (Simmons, 2010). Encourage the patient to verbalize feelings about the illness and its effects, if possible, while fostering hope. Assess previous decision-making patterns, roles, and responsibilities. To help identify personal factors that influence coping ability, ask the patient and/or family to describe their usual lifestyles and the situations in which they coped effectively. Sleep disturbances related to pain and altered autonomic function may affect the patient’s sleep-wake cycle. Allow for regularly scheduled rest periods (Simmons, 2010).
Refer patients who need further psychosocial support to the social worker, certified hospital chaplain or appropriate spiritual resource, and local support groups. If necessary, obtain a psychological consultation for further evaluation and intervention.
Community-Based Care
The severity and course of Guillain-Barré syndrome (GBS) are extremely variable, which makes the prognosis difficult to predict. The most likely residual deficits at discharge are related to motor status and mobility, self-management, and possibly sensory alteration and disturbed self-concept. For patients who have total quadriparesis (weakness in all four extremities) or respiratory paralysis, the course of the rehabilitation phase is even more variable and may require weeks to years. The expected outcome of the recovery phase is to move from dependence to independence, if possible.
Planning for discharge begins on admission. The patient may be discharged to home or to a rehabilitation unit. In collaboration with the discharge planner or case manager (CM), the nurse makes appropriate referrals to a rehabilitation setting, home care agency, and/or community agencies for assistance in the home setting after discharge.
Include a family member in the education process throughout the patient’s hospitalization and in the discharge process. Provide them with both oral and written instructions to improve mobility, use adaptive-assistive devices, and prevent skin breakdown.
If the patient is discharged to home while still needing assistive devices, the CM in collaboration with the interdisciplinary health care team makes certain that the necessary equipment has been delivered after evaluating the home setting. Home care management for patients with GBS is similar to that for those who have had a stroke or spinal cord injury, depending on the nature of the neurologic deficit.
Self-help and support groups for patients with chronic illness are common. Refer the patient and family to these groups, if indicated. For example, the Guillain-Barré Syndrome Foundation International (www.gbs-cidp.org) provides resources and information for patients and their families. The psychosocial adjustment needed may be minimal or dramatic, depending on the patient’s residual deficit, age, gender, usual roles and responsibilities, usual coping strategies, available support systems, and occupation. Help the patient identify other support systems, such as church members, friends, or spiritual resources.
Myasthenia Gravis
Pathophysiology
Myasthenia gravis (MG) is an acquired autoimmune disease characterized by fatigue and weakness primarily in muscles innervated by the cranial nerves, as well as in skeletal and respiratory muscles. This autoimmune disease of the neuromuscular junction may take many forms—from mild disturbances of the ocular muscles to a rapidly developing, generalized weakness that may lead to death from respiratory failure. It has remissions and exacerbations (worsening or “flare-ups”). Although MG can present at any age, it is less commonly seen in the United States than Guillain-Barré syndrome. The incidence is equal between men and women.
MG is caused by an autoantibody attack on the acetylcholine receptors (AChRs) in the muscle end plate membranes. As a result, nerve impulses are not transmitted to the skeletal muscle at the neuromuscular junction and the muscles cannot contract.
Although the disease is not hereditary, there is a small familial incidence. Evidence also suggests a relationship between MG and hyperplasia (overgrowth) of the thymus gland. The thymus gland is often abnormal. Thymoma (encapsulated thymus gland tumor) occurs in a few cases, but most of the remaining cases show hyperplasia of the thymus. There is also a very strong association between MG and hyperthyroidism. D-penicillamine, interferon-alpha, and bone marrow transplantation have been associated with drug-induced (iatrogenic) autoimmune MG.
Patient-Centered Collaborative Care
Assessment
Physical Assessment/Clinical Manifestations
Although the onset of MG is usually insidious (slow), some instances of fairly rapid development have been caused by infection, pregnancy, or anesthesia. Ask about any history of these events. A temporary increase in weakness may be noted after vaccination, menstruation, and exposure to extremes in environmental temperature.
In addition to the biographic data and history, ask the patient if he or she noticed the rapid onset of fatigue. Note reports of muscle weakness that increases on exertion or as the day wears on and improves with rest. Ask the patient to describe his or her symptoms, specifically noting the affected muscle groups and any limitation or inability in performing ADLs.
Additional areas of inquiry include any history of ptosis (drooping eyelids), diplopia (double vision), or dysphagia (difficulty chewing or swallowing) and the type of diet best tolerated. Assess the patient about a history of respiratory difficulty, choking, or voice weakness. Other areas of assessment include asking about any difficulty holding up the head, brushing teeth, combing hair, or shaving. Inquiry is made about the presence of paresthesias or aching in weakened muscles. Finally, ask about a history of thymus gland tumor.
The most common symptoms are related to involvement of the levator palpebrae or extraocular muscles (Chart 46-3). Assess for ptosis, diplopia, and weak or incomplete eye closure. These symptoms may last only a few days at the onset and then resolve, only to return weeks or months later. Pupillary responses to light and accommodation are usually normal.