M. Linda Workman
Care of Patients with Pituitary and Adrenal Gland Problems
Learning Outcomes
Safe and Effective Care Environment
Health Promotion and Maintenance
Psychosocial Integrity
Physiological Integrity
11 Coordinate nursing care for the patient immediately after a transsphenoidal hypophysectomy.
13 Compare the clinical manifestations of hypercortisolism and adrenal insufficiency.
14 Prioritize nursing care for the patient with acute adrenal insufficiency.
15 Coordinate nursing care for the patient with Cushing’s disease or syndrome.

http://evolve.elsevier.com/Iggy/
Answer Key for NCLEX Examination Challenges and Decision-Making Challenges
Audio Glossary
Concept Map Creator
Key Points
Review Questions for the NCLEX® Examination
Hormones secreted from the pituitary gland and adrenal glands influence the function of many tissues and organs. When any of these glands secrete too much or too little of one or more hormones, the effects are widespread and may induce psychological, as well as physical, changes. The anterior pituitary gland regulates growth, metabolism, pigmentation, and sexual development. The posterior pituitary gland secretes vasopressin, also known as antidiuretic hormone or ADH. Posterior pituitary problems result in fluid and electrolyte imbalances. The adrenal gland produces and secretes hormones that influence homeostasis and are life sustaining. Nursing care for the patient with pituitary or adrenal gland disorders includes assessment, patient education, evaluation of patient response to therapy, and providing support.
A complete history and physical examination are performed to detect specific clinical findings. The patient also often undergoes many diagnostic tests and relies on the nurse for specific instructions and explanations. Surgical intervention may be indicated. The patient often needs lifelong hormone replacement therapy, and physical and emotional support are critical.
Disorders of the Anterior Pituitary Gland
The anterior pituitary gland (adenohypophysis) controls growth, metabolic activity, and sexual development through the actions of these hormones:
• Growth hormone (GH; somatotropin)
• Thyrotropin (thyroid-stimulating hormone [TSH])
• Corticotropin (adrenocorticotropic hormone [ACTH])
• Follicle-stimulating hormone (FSH)
• Melanocyte-stimulating hormone (MSH)
Hormone disorders of the anterior pituitary gland can result from problems arising within the anterior pituitary gland itself (primary pituitary dysfunction) or from problems in the hypothalamus that change anterior pituitary function (secondary pituitary dysfunction). In either case, one or more hormones may be undersecreted (pituitary hypofunction) or oversecreted (pituitary hyperfunction).
Hypopituitarism
Pathophysiology
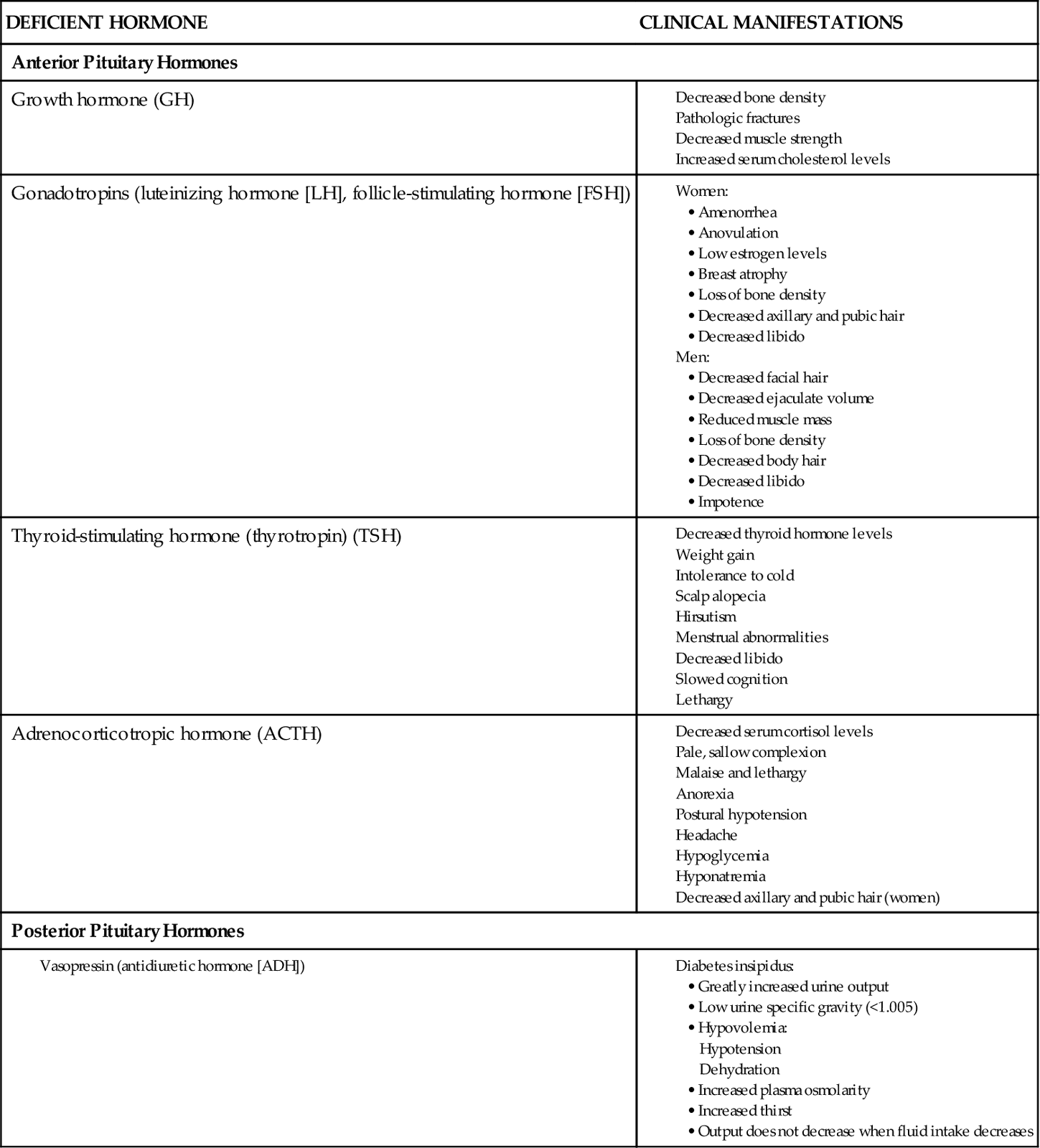
A person with hypopituitarism has a deficiency of one or more anterior pituitary hormones, resulting in metabolic problems and sexual dysfunction. If only one hormone is affected, the condition is known as selective hypopituitarism. Decreased production of all of the anterior pituitary hormones is an extremely rare condition known as panhypopituitarism.
More commonly, there is a decrease in the secretion of one hormone and a lesser decrease in the secretion of other hormones. Deficiencies of adrenocorticotropic hormone (ACTH) and thyroid-stimulating hormone (TSH) are the most life threatening because they cause a decrease in the secretion of vital hormones from the adrenal and thyroid glands. Adrenal gland hypofunction is discussed on pp. 1381-1384; hypothyroidism is discussed in Chapter 66.
Deficiency of the gonadotropins (luteinizing hormone [LH] and follicle-stimulating hormone [FSH]—hormones that stimulate the ovaries and testes to produce sex hormones) changes sexual function in both men and women. In men, gonadotropin deficiency results in testicular failure, with decreased testosterone production from the Leydig cells and decreased or absent sperm production. Decreased testosterone levels in men cause sterility. In women, gonadotropin deficiency results in ovarian failure, amenorrhea, and infertility.
Growth hormone (GH) deficiency changes tissue growth patterns indirectly. GH itself has little effect on tissues and cells; however, it stimulates the liver to produce somatomedins. These substances, especially somatomedin C, then enhance growth activities in cells and tissues. Somatomedin C is responsible for bone and cartilage growth and maintenance.
GH deficiency may be a result of decreased GH production, failure of the liver to produce somatomedins, or a failure of the cells or tissues to respond to the somatomedins. Deficiency in children leads to short stature and other manifestations of growth retardation. Deficiency in adults does not affect height but does increase the rate of bone destructive activity, leading to thinner, more fragile bones (osteoporosis) and an increased risk for fractures.
The cause of hypopituitarism varies. Benign or malignant pituitary tumors can compress and destroy pituitary tissue. Pituitary function can be impaired by severe malnutrition or rapid loss of body fat, such as in people with anorexia nervosa (a disorder in which people see themselves as overweight and eat so little that starvation results). Shock or severe hypotension reduces blood flow to the pituitary gland, leading to hypoxia and infarction. Other causes of hypopituitarism include head trauma, brain tumors or infection, radiation or surgery of the head and brain, and acquired immune deficiency syndrome (AIDS) (Melmed & Kleinberg, 2008). Idiopathic hypopituitarism is an isolated hormone deficiency with an unknown cause.
Postpartum hemorrhage is the most common cause of pituitary infarction, which results in decreased hormone secretion. This clinical problem is known as Sheehan’s syndrome. The pituitary gland normally enlarges during pregnancy, and when hypotension results from hemorrhage, ischemia and necrosis of the gland occur. Usually this condition develops immediately after delivery, although some cases have occurred several years later.
Patient-Centered Collaborative Care
Assessment
Changes in physical appearance and target organ function occur with deficiencies of specific pituitary hormones (Chart 65-1). Gonadotropin (LH and FSH) deficiency results in the loss of or change in secondary sex characteristics in men and women. While assessing the male patient, look for facial and body hair loss. Ask about episodes of impotence and decreased libido (sex drive). Women may report amenorrhea (absence of menstrual periods), dyspareunia (painful intercourse), infertility, and decreased libido. While examining the female patient, check for dry skin, breast atrophy, and a decreased amount or absence of axillary and pubic hair.
Neurologic manifestations of hypopituitarism as a result of tumor growth often first occur as changes in vision. Assess the patient’s visual acuity, especially peripheral vision, for changes or loss. Temporal headaches are a common finding. Other manifestations may include diplopia (double vision) and ocular muscle paralysis, limiting eye movement.
Laboratory findings vary widely with hypopituitarism. Some pituitary hormone levels may be measured directly. Often, however, the effects of the hormones, rather than their actual levels, are assessed. Blood levels of triiodothyronine (T3) and thyroxine (T4) from the thyroid, as well as testosterone and estradiol from the gonads, are measured easily. If levels of one or all of these hormones are low or in the low-normal range, further evaluation is necessary. Levels of pituitary gonadotropins (LH and FSH) and TSH are sufficient if function of the target organ is apparent. Function of LH and FSH is assessed by observing for the presence of secondary sexual characteristics. Function of TSH is assessed by measuring circulating levels of thyroid hormones. ACTH levels may be normal or low, and prolactin (PRL) levels are low to high.
Stimulation testing can be useful to evaluate pituitary function. For example, insulin injection in people with normal pituitary function causes an increased release of GH and ACTH. With decreased pituitary function, levels of either of these hormones remain unchanged.
Pituitary problems may cause changes in the sella turcica (the bony nest where the pituitary gland rests) that can be seen with skull x-rays. Changes may include enlargement, erosion, and calcifications as a result of pituitary tumors. Computed tomography (CT) and magnetic resonance imaging (MRI) can more distinctly define bone or soft-tissue lesions. An angiogram may be used to rule out the presence of an aneurysm or other vascular problems in the area before surgery.
Interventions
Management of the adult with hypopituitarism focuses on replacement of deficient hormones. Older patients or those with a chronic disease often require a lower amount of hormone replacement. Men who have gonadotropin deficiency receive sex steroid replacement therapy with androgens (testosterone). The most effective route of androgen replacement is IM, although use of transdermal testoste-rone patches is increasing. Instruct the patient in self-administration. Therapy begins with high-dose testosterone and is continued until virilization (presence of male secondary sex characteristics) is achieved. Maximal effects of treatment include increases in penis size, libido, muscle mass, bone size, and bone strength. Chest, facial, pubic, and axillary hair growth also increases, and the voice deepens. Patients usually report improved self-esteem and body image after therapy is initiated. The dose may then be decreased, but therapy continues throughout life.
Androgen therapy is avoided in men with prostate cancer. Side effects of testosterone therapy include gynecomastia (male breast tissue development), acne, baldness, and prostate enlargement.
Achieving fertility in these patients is difficult and requires additional parenteral testosterone therapy and injections of human chorionic gonadotropin (hCG). Teach the patient about the additional therapy, and provide emotional support because the outcome of fertility treatment is uncertain.
Women who have gonadotropin deficiency receive hormone replacement with a combination of estrogen and progesterone. The risk for hypertension or thrombosis (formation of blood clots in deep veins) is increased with estrogen therapy, especially among women who smoke. Emphasize measures to reduce risk and the need for regular health visits. For women who wish to become pregnant, clomiphene (Clomid) may be given to induce ovulation. Gonadotropin-releasing hormone (GnRH) and hCG are used to stimulate ovulation if therapy with clomiphene fails.
Adult patients with GH deficiency may be treated with injections of GH, although this treatment is rare.
Hyperpituitarism
Pathophysiology
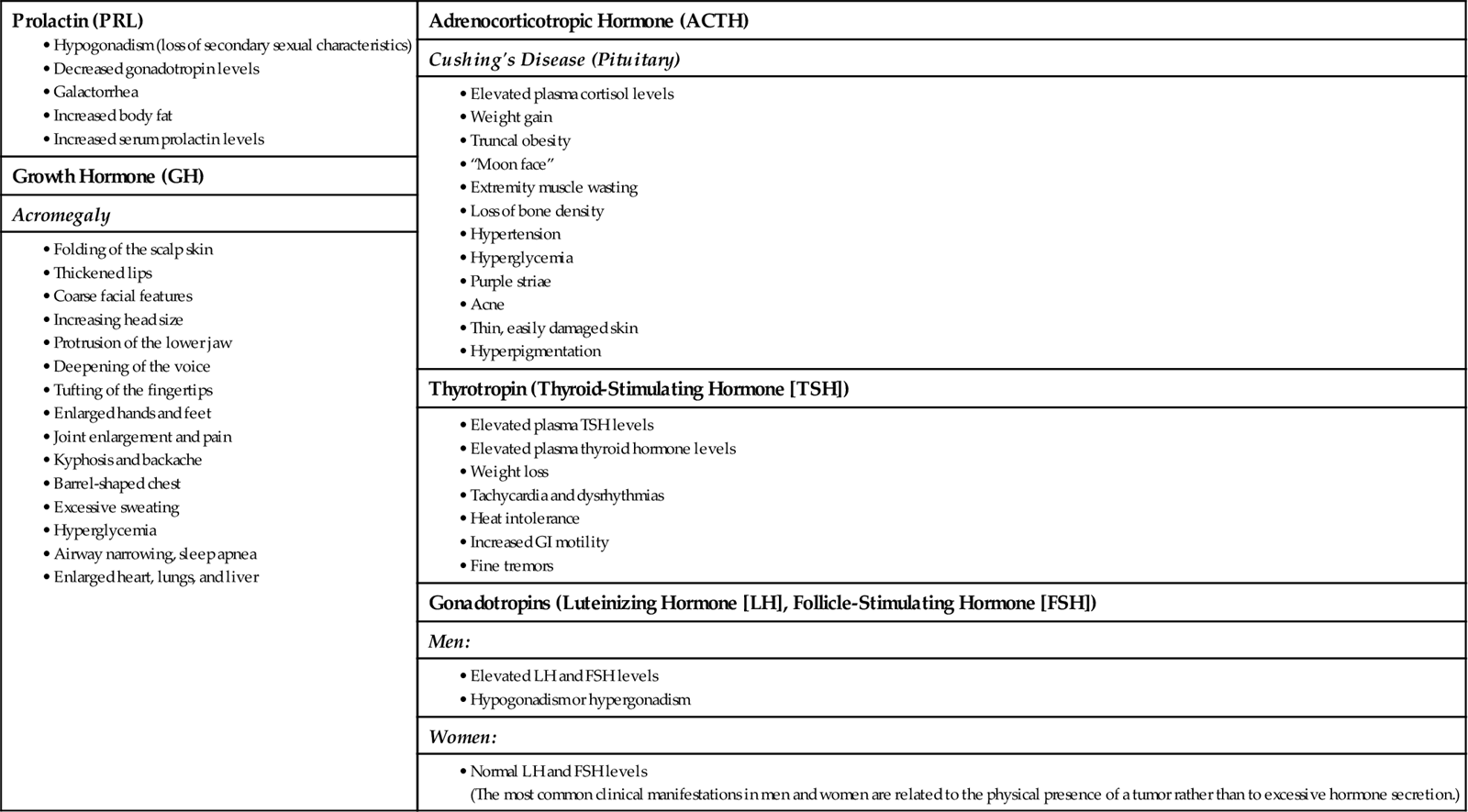
Hyperpituitarism is hormone oversecretion that occurs with pituitary tumors or tissue hyperplasia (tissue overgrowth). Tumors occur most often in the anterior pituitary cells that produce growth hormone (GH), prolactin (PRL), and adrenocorticotropic hormone (ACTH). Overproduction of PRL also may occur in response to tumors that overproduce GH and ACTH. Excess ACTH may occur with increased secretion of melanocyte-stimulating hormone (MSH).
The most common cause of hyperpituitarism is a pituitary adenoma—a benign tumor of one or more tissues within the anterior pituitary. Adenomas are classified by size, invasiveness, and the hormone secreted. An invasive pituitary adenoma involves a portion or all of the sella turcica. When the sella turcica is not involved, the adenoma is “enclosed.”
As an adenoma gets larger and compresses brain tissue, neurologic symptoms, as well as endocrine symptoms, may occur. Such symptoms may include visual changes, headache, and increased intracranial pressure (ICP).
Prolactin (PRL)-secreting tumors are the most common type of pituitary adenoma. Excessive PRL inhibits the secretion of gonadotropins and sex hormones in men and women, resulting in galactorrhea (breast milk production), amenorrhea, and infertility.
Overproduction of GH in adults results in acromegaly (Fig. 65-1). The onset may be gradual with slow progression, and changes may remain unnoticed for years before diagnosis of the disorder. Early detection and treatment are essential to prevent irreversible changes in the soft tissues, such as those of the face, hands, feet, and skin. Other changes include increased skeletal thickness, hypertrophy of the skin, and enlargement of many organs, such as the liver and heart. Some changes may be reversible after treatment, but skeletal changes are permanent.

Bone thinning and bone cell overgrowth occur slowly. Degeneration of joint cartilage and hypertrophy of ligaments, vocal cords, and eustachian tubes are common. Nerve entrapment occurs because of tissue overgrowth and myelin loss in peripheral nerves. Because GH blocks the action of insulin, hyperglycemia (elevated blood glucose levels) is also common.
Excess ACTH overstimulates the adrenal cortex. The result is excessive production of glucocorticoids, mineralocorticoids, and androgens, which leads to the development of Cushing’s disease (see Hypercortisolism [Cushing’s Disease], pp. 1384-1390).
Usually, hyperpituitarism is caused by hormone-secreting benign tumors (adenomas) arising from one pituitary cell type. It can also be caused by hypothalamic problems that lead to excessive production of releasing hormones, which then overstimulate the normal pituitary gland.
Patient-Centered Collaborative Care
Assessment
The manifestations of hyperpituitarism vary, depending on which hormone is produced in excess. Obtain data about the patient’s age, gender, and family history. Ask about any change in hat, glove, ring, or shoe size. Fatigue and lethargy are common. The patient with high GH levels may have backache and arthralgias (joint pain) from bone changes. Ask specifically about headaches and changes in vision.
The patient with hypersecretion of PRL often reports difficulties in sexual functioning. Ask women about menstrual changes (e.g., amenorrhea, irregular menses, and difficulty in becoming pregnant) and about decreased libido or dyspareunia (painful intercourse). Men may report decreased libido and impotence.
Changes in appearance and target organ function occur with excesses of specific anterior pituitary hormones (Chart 65-2). Initial manifestations of GH excess are increases in lip and nose sizes, a prominent brow ridge, and increases in head, hand, and foot sizes. The patient with hyperpituitarism often seeks health care because of dramatic changes in appearance. Assess the impact of these changes on self-image and personal relationships.
In hyperpituitarism, usually only one hormone is produced in excess because the cell types within the pituitary gland are so individually organized. The most common hormones produced in excess are PRL, ACTH, and GH. Tumors producing TSH, luteinizing hormone (LH), or follicle-stimulating hormone (FSH) are rare, and elevations of these hormones warrant evaluation. Elevations of LH and FSH are normal in the postmenopausal woman.
Imaging assessment for hyperpituitarism is the same as for hypopituitarism. Skull x-rays are used to identify abnormalities of the sella turcica. CT scans and MRI can define soft-tissue lesions, and angiography can rule out an aneurysm or vascular malformations.
Suppression testing can help diagnose hyperpituitarism and determine whether the normal negative feedback control mechanisms for hormonal regulation are intact. For example, high blood glucose levels suppress the release of GH. In a suppression test, 100 g of oral glucose or 0.5 g/kg of body weight is given IV. GH levels are measured serially for up to 120 minutes. GH levels that do not fall below 5 ng/mL indicate a positive (abnormal) result.
Interventions
The expected outcomes of management for the patient who has hyperpituitarism are to return hormone levels to normal or near normal, reduce or eliminate headache and visual disturbances, prevent complications, and reverse as many of the body changes as possible.
Nonsurgical Management
Encourage the patient to express concerns about his or her altered physical appearance. Help him or her identify personal strengths and positive characteristics, reinforcing each patient’s uniqueness and importance. Galactorrhea, gynecomastia, and reduced sexual functioning can disturb self-image and personal identity. Reassure the patient that treatment may reverse some of these problems. Encourage the patient to discuss his or her feelings.
Drug therapy may be used alone or in combination with surgery and/or radiation. The most common drugs used are dopamine agonists, including bromocriptine mesylate (Parlodel), cabergoline (Dostinex), and pergolide (Permax) (Melmed & Kleinberg, 2008). These drugs stimulate dopamine receptors in the brain and inhibit the release of GH and PRL. In most cases, small tumors decrease until the pituitary gland is of normal size. Large pituitary tumors usually decrease to some extent. In patients with acromegaly, bromocriptine reduces GH levels and decreases tumor size, especially when GH levels remain high after surgery or before the full effect of radiation therapy has occurred.
Side effects of bromocriptine include orthostatic (postural) hypotension, gastric irritation, nausea, headaches, abdominal cramps, and constipation. Give bromocriptine with a meal or a snack to reduce some of these side effects. Treatment starts with a low dose and is gradually increased until the desired level (usually 7.5 mg/day) is reached. If pregnancy occurs, the drug is stopped immediately.
Other agents used for acromegaly are the somatostatin analogs, especially octreotide (Sandostatin), and a growth hormone receptor blocker, pegvisomant (Somavert). Octreotide inhibits GH release through negative feedback. Pegvisomant blocks growth hormone (GH) receptor activity and production of insulin-like growth factor (IGF). Although these therapies are effective, a disadvantage is that they must be given as an injection on a daily or weekly schedule. A major side effect is gallbladder disease. Pegvisomant may cause an increase in tumor size.
Radiation therapy is not useful in the immediate management of acute hyperpituitarism. These therapy regimens take a long time to complete, and several years may pass before a therapeutic effect can be seen. Side effects of radiation therapy include hypopituitarism, optic nerve damage, and other eye and vision problems. Use of the gamma knife procedure is increasing the accuracy of radiation therapy.
Surgical Management
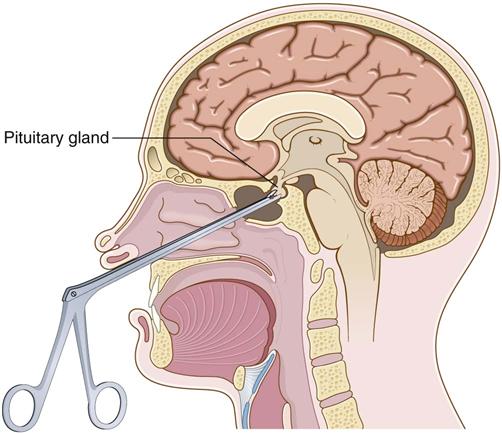
Surgical removal of the pituitary gland and tumor (hypophysectomy) is the most common treatment for hyperpituitarism.
Preoperative Care.
Explain that hypophysectomy decreases hormone levels, relieves headaches, and may reverse changes in sexual functioning. Body changes, organ enlargement, and visual changes are not usually reversible. Explain that because nasal packing is present for 2 to 3 days after surgery, it will be necessary to breathe through the mouth, and a “mustache” dressing (“drip” pad) will be placed under the nose. Instruct the patient not to brush teeth, cough, sneeze, blow the nose, or bend forward after surgery. These activities can increase intracranial pressure (ICP) and delay healing.
Operative Procedures.
Depending on tumor size and other patient factors, a minimally invasive endoscopic transnasal approach may be used instead of the more traditional transsphenoidal approach (Fig. 65-2). Although both procedures are less invasive than a craniotomy, the endoscopic approach uses smaller diameter instruments and results in less damage to nasal structures (Graham et al., 2009). Both procedures are performed with the patient in a semi-sitting position. For the transsphenoidal approach, the surgeon makes an incision just above the upper lip and reaches the pituitary gland through the sphenoid sinus. After the gland is removed, a muscle graft is taken, often from the thigh, to support the area and prevent leakage of cerebrospinal fluid (CSF). Nasal packing is inserted after the incision is closed, and a mustache dressing is applied. If the tumor cannot be reached by either the endoscopic transnasal approach or the transsphenoidal approach, a craniotomy may be indicated (see Chapter 47).
Postoperative Care.
Monitor the patient’s neurologic response, and document any changes in vision or mental status, altered level of consciousness, or decreased strength of the extremities. Observe the patient for complications such as transient diabetes insipidus (discussed on pp. 1378-1380), CSF leakage, infection, and increased ICP.
Teach the patient to report any postnasal drip or increased swallowing, which might indicate leakage of CSF. Keep the head of the bed elevated after surgery. Assess nasal drainage for quantity, quality, and the presence of glucose (which indicates that the fluid is CSF). A light yellow color at the edge of the clear drainage on the dressing is called the “halo sign” and indicates CSF. If the patient has persistent, severe headaches, CSF fluid may have leaked into the sinus area. Most CSF leaks resolve with bedrest. If the CSF leak persists, the physician may perform a spinal tap to reduce CSF pressure. Surgical intervention is rarely necessary.
Teach the patient to avoid coughing early after surgery because it increases pressure in the incision area and may lead to a CSF leak. Remind the patient to perform deep-breathing exercises hourly while awake to prevent pulmonary problems. Patients may have mouth dryness from mouth breathing. Perform frequent oral rinses, and apply a lubricating jelly to dry lips.
Infection can occur after surgery. Specifically assess for manifestations of meningitis, such as headache, fever, and nuchal (neck) rigidity. The surgeon may prescribe antibiotics, analgesics, and antipyretics.
If the entire pituitary gland has been removed, replacement of thyroid hormones and glucocorticoids is lifelong. Best practices for care after surgery are listed in Chart 65-3.
After surgery, the patient needs daily self-management regimens and frequent checkups. He or she may need strategies to reduce stress. The home care nurse performs a focused assessment during any home visit to a patient who has undergone a hypophysectomy (Chart 65-4). Review drug regimens and manifestations of infection and cerebral edema with the family.
After a transsphenoidal hypophysectomy, advise the patient to avoid activities that might interfere with healing. Teach him or her to avoid bending over from the waist to pick up objects or tie shoes because this position increases ICP. Teach the patient to bend the knees and then lower the body to pick up fallen objects. ICP also increases when the patient strains to have a bowel movement. Suggest techniques to prevent constipation, such as eating high-fiber foods, drinking plenty of fluids, and using stool softeners or laxatives. Activities that increase ICP should be avoided for up to 2 months after surgery.
Teach the patient to avoid toothbrushing for about 2 weeks after surgery until the incision has healed. Frequent mouth care (every 4 to 6 hours) with mouthwash and daily flossing provide adequate oral hygiene. Numbness in the area of the incision and a decreased sense of smell are expected after surgery and usually last 3 to 4 months. Advise the patient to use a mirror to check the gums for bleeding, because reduced sensation increases the risk for injury.
After a hypophysectomy, hormone replacement with vasopressin may be needed to maintain fluid balance. (See discussion of Interventions on pp. 1378-1380 in the Diabetes Insipidus section.) If the anterior portion of the pituitary gland is removed, instruct the patient in cortisol, thyroid, and gonadal hormone replacement. Teach the patient to report the return of any symptoms of hyperpituitarism immediately to the primary health care provider.
Disorders of the Posterior Pituitary Gland
Disorders of the posterior pituitary gland (neurohypophysis) are related to a deficiency or excess of the hormone vasopressin (antidiuretic hormone [ADH]) and usually occur independently from anterior pituitary problems. Diabetes insipidus occurs with ADH deficiency, and the syndrome of inappropriate antidiuretic hormone (SIADH) occurs with ADH excess.
Diabetes Insipidus
Pathophysiology
Diabetes insipidus (DI) is a water metabolism problem caused by an ADH deficiency (either a decrease in ADH synthesis or an inability of the kidneys to respond to ADH). ADH deficiency results in the excretion of large volumes of dilute urine. Without ADH, distal kidney tubules and collecting ducts do not reabsorb water, leading to polyuria (excessive water loss through urination) and dehydration.
Dehydration caused by this massive water loss increases plasma osmolarity, which stimulates the osmoreceptors to relay a sensation of thirst to the cerebral cortex. Thirst promotes increased fluid intake and aids in maintaining water homeostasis. If the thirst mechanism is poor or absent or if the person is unable to obtain water, dehydration becomes more severe and can lead to death.
ADH deficiency is classified as nephrogenic, drug-related, primary, or secondary, depending on whether the problem is caused by insufficient production of ADH or an inability of the kidney to respond to the presence of ADH (Simmons, 2010).
Nephrogenic diabetes insipidus is an inherited disorder. The kidney tubules do not respond to the actions of ADH, which results in poor water reabsorption by the kidney. The actual amount of hormone produced is not deficient.
Primary diabetes insipidus is caused by a defect in the hypothalamus or pituitary gland, resulting in a lack of ADH production or release. Secondary diabetes insipidus can result from tumors in or near the hypothalamus or pituitary gland, head trauma, infectious processes, surgical procedures (hypophysectomy), or metastatic tumors. Less often, it is caused by brain hemorrhage, brain disease, or cerebral aneurysm, which reduces ADH production.
Drug-related diabetes insipidus is usually caused by lithium carbonate (Eskalith, Lithobid, Carbolith ![]() ) and demeclocycline (Declomycin). These drugs can interfere with the response of the kidneys to ADH.
) and demeclocycline (Declomycin). These drugs can interfere with the response of the kidneys to ADH.
Patient-Centered Collaborative Care
Assessment
Most manifestations of DI are related to dehydration (Chart 65-5) (Simmons, 2010). The key manifestations are an increase in the frequency of urination and excessive thirst. Ask about a history of any known causative factors, such as recent surgery, head trauma, or drug use (e.g., lithium). Although increased fluid intake prevents serious volume depletion, the patient who is deprived of fluids or who cannot increase oral fluid intake may develop shock from fluid loss. Manifestations of dehydration, such as poor skin turgor and dry or cracked mucous membranes or skin, may be present. (See Chapter 13 for further discussion of dehydration.)