Donna D. Ignatavicius
Care of Patients with Musculoskeletal Trauma
Learning Outcomes
Safe and Effective Care Environment
Health Promotion and Maintenance
Psychosocial Integrity
Physiological Integrity
9 Compare and contrast common types of fractures.
10 Describe the usual healing process for bone.
11 Assess patients with musculoskeletal trauma to prioritize interventions for their care.
12 Explain the typical clinical manifestations that are seen in patients with fractures.
13 Delineate nursing care needed to maintain casts for patients with fractures.
15 Plan pain management for patients with musculoskeletal trauma.
16 Identify the risk for complications from fractures, and take measures to help prevent them.
19 Describe emergency care for people who have a traumatic amputation.
20 Plan postoperative care, including health teaching, after an elective amputation.
21 Identify common causes of amputations.
22 Identify appropriate complementary and alternative therapies for patients with phantom limb pain.
23 Describe the patient-centered collaborative care needed to manage complex regional pain syndrome.
24 Plan care for patients with common types of sports-related injuries.

http://evolve.elsevier.com/Iggy/
Answer Key to NCLEX Examination Challenges and Decision-Making Challenges
Audio Glossary
Concept Map Creator
Key Points
Review Questions for the NCLEX® Examination
Musculoskeletal trauma accounts for about two thirds of all injuries and is one of the primary causes of disability in the United States. It ranges from simple muscle strain to multiple bone fractures with severe soft-tissue damage.
Fractures and other musculoskeletal trauma impair a patient’s mobility in varying degrees, depending on the severity and extent of the injury. These injuries also affect sensation because of pressure on nerve endings from edema. In some cases, peripheral nerves are directly damaged as a result of musculoskeletal injury.
Fractures
Pathophysiology
A fracture is a break or disruption in the continuity of a bone that often affects mobility and sensation. It can occur anywhere in the body and at any age. All fractures have the same basic pathophysiologic mechanism and require similar patient-centered collaborative care, regardless of fracture type or location.
Classification of Fractures
A fracture is classified by the extent of the break:
A fracture is described by the extent of associated soft-tissue damage as open (or compound) or closed (or simple). The skin surface over the broken bone is disrupted in a compound fracture, which causes an external wound. These fractures are often graded to define the extent of tissue damage. A simple fracture does not extend through the skin and therefore has no visible wound.
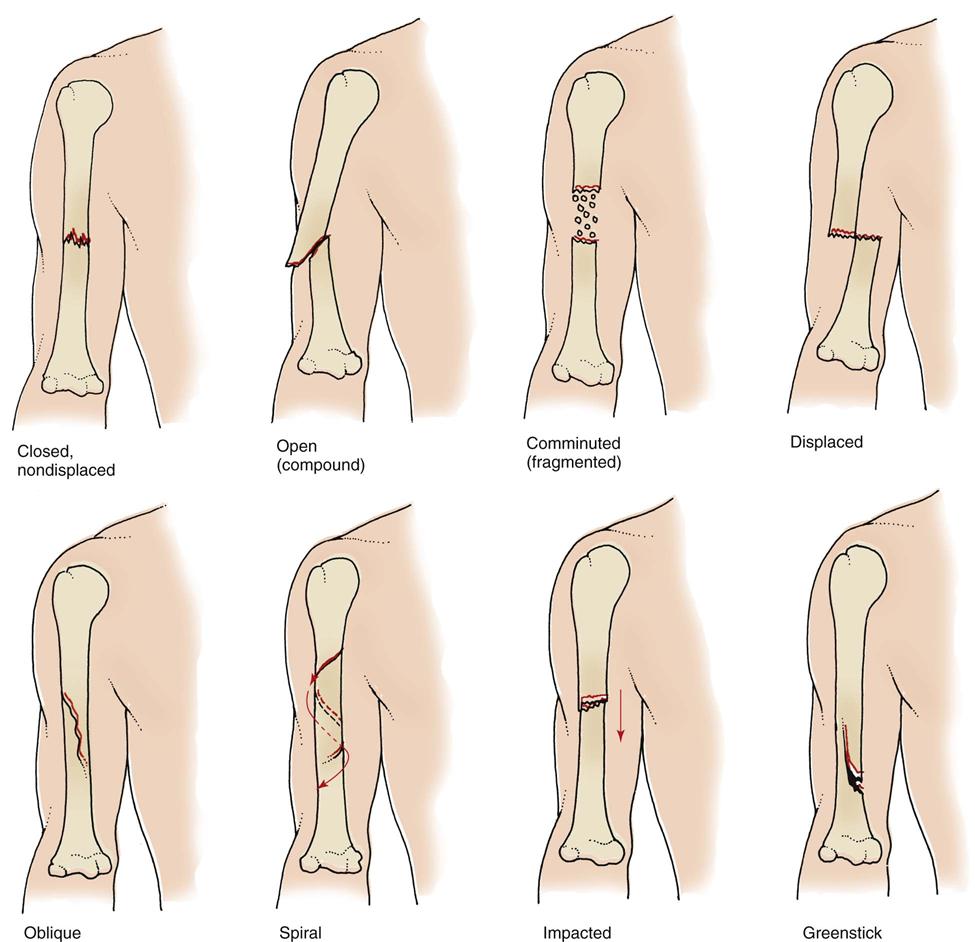
Fig. 54-1 shows common types of fractures. In addition to being identified by type, fractures are described by their cause. A pathologic (spontaneous) fracture occurs after minimal trauma to a bone that has been weakened by disease. For example, a patient with bone cancer or osteoporosis can easily have a pathologic fracture. A fatigue (stress) fracture results from excessive strain and stress on the bone. This problem is commonly seen in recreational and professional athletes. Compression fractures are produced by a loading force applied to the long axis of cancellous bone. They commonly occur in the vertebrae of older patients with osteoporosis and are extremely painful.
Stages of Bone Healing
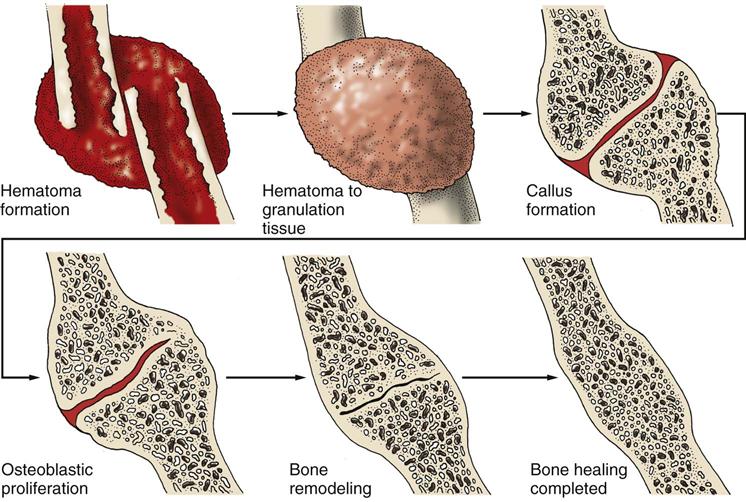
When a bone is fractured, the body immediately begins the healing process to repair the injury and restore the body’s equilibrium. Fractures heal in five stages that are a continuous process and not single stages. In stage one, within 24 to 72 hours after the injury, a hematoma forms at the site of the fracture because bone is extremely vascular. Stage two occurs in 3 days to 2 weeks when granulation tissue begins to invade the hematoma. This then prompts the formation of fibrocartilage, providing the foundation for bone healing. Stage three of bone healing occurs as a result of vascular and cellular proliferation. The fracture site is surrounded by new vascular tissue known as a callus (within 3 to 6 weeks). Callus formation is the beginning of a nonbony union. As healing continues in stage four, the callus is gradually resorbed and transformed into bone. This stage usually takes 3 to 8 weeks. During the fifth and final stage of healing, consolidation and remodeling of bone continue to meet mechanical demands. This process may start as early as 4 to 6 weeks after fracture and can continue for up to 1 year, depending on the severity of the injury and the age and health of the patient. Fig. 54-2 summarizes the stages of bone healing.
In young, healthy adult bone, healing takes about 4 to 6 weeks. In the older person who has reduced bone mass, healing time is lengthened. Complete healing often takes 3 to 6 months or longer in people who are older than 70 years. Other factors also affect healing. Examples include the severity of the trauma, the type of bone injured, how the fracture is managed, infections at the fracture site, and ischemic or avascular necrosis (AVN).
Complications of Fractures
Regardless of the type or location of the fracture, several limb- and life-threatening acute and chronic complications can result from the injury. Clinical manifestations of beginning complications must be treated early to prevent serious consequences. In some cases, careful monitoring and assessment can prevent these complications:
Acute Compartment Syndrome.
Compartments are areas in the body in which muscles, blood vessels, and nerves are contained within fascia. Most compartments are located in the extremities. Fascia is an inelastic tissue that surrounds groups of muscles, blood vessels, and nerves in the body. Acute compartment syndrome (ACS) is a serious condition in which increased pressure within one or more compartments reduces circulation to the area. The most common sites for this problem in patients with musculoskeletal trauma are the compartments in the lower leg and forearm.
The pathophysiologic changes of increased compartment pressure are sometimes referred to as the ischemia-edema cycle. Capillaries within the muscle dilate, which raises capillary pressure. Capillaries then become more permeable because of the release of histamine by the ischemic muscle tissue. As a result, plasma proteins leak into the interstitial fluid space and edema occurs. Edema increases pressure on nerve endings and causes pain. Blood flow to the area is reduced, and further ischemia results. Sensory deficits or paresthesia generally appears before changes in vascular or motor signs. The color of the tissue pales, and pulses begin to weaken but rarely disappear. The affected area is usually palpably tense, and pain occurs with passive motion of the extremity. If the condition is not treated, cyanosis, tingling, numbness, paresis, necrosis, and severe pain can occur. Chart 54-1 summarizes the sequence of pathophysiologic events in compartment syndrome and the associated clinical assessment findings.
The pressure to the compartment can be from an external or internal source. Tight, bulky dressings and casts are examples of external pressure. Blood or fluid accumulation in the compartment is a common source of internal pressure. The injury or trauma causing the problem is above the compartment involved, which decreases blood flow to the more distal area of injury. ACS is not limited to patients with musculoskeletal problems. It can also occur in those with severe burns, extensive insect bites or snakebites, or massive infiltration of IV fluids. In these situations, edema increases internal pressure in one or more compartments.
Problems resulting from compartment syndrome include infection, persistent motor weakness in the affected extremity, contracture, and myoglobinuric renal failure. In extreme cases, amputation becomes necessary.
Infection from necrosis may become severe enough that amputation of the limb is needed. Motor weakness from injured nerves is not reversible, and the patient may require an orthotic device for assistance in mobility. Volkmann’s contractures of the forearm, which can begin within 12 hours of the pressure increase, result from shortening of the ischemic muscle and from nerve involvement.
Myoglobinuric renal failure from muscle breakdown is a potentially fatal complication of compartment syndrome. It occurs when large or multiple compartments are involved. Injured muscle tissues release myoglobulin (muscle protein) into the circulation, where it can clog the renal tubules and cause acute renal failure. Although the exact pathophysiologic mechanisms are unknown, it is suspected that myoglobulin has a direct toxic effect on the kidney. Damaged muscle cells also release potassium, which cannot be excreted because of the renal failure. The resulting hyperkalemia may cause dysrhythmias and cardiac arrest.
Crush Syndrome.
Crush syndrome (CS) occurs from an external crush injury that compresses one or more compartments in the leg, arm, or pelvis. It is a potentially life-threatening, systemic complication that results from hemorrhage and edema after a severe fracture injury. As muscle becomes ischemic and necrotic from pressure within the compartment, myoglobin is released into circulation, where it can occlude the distal renal tubules and result in kidney failure.
Specific causes of CS include:
• Natural disasters, such as earthquakes
• Work-related injuries, such as being trapped under heavy equipment such as a car
• Older adults who fall, are unable to get up, and lie for a prolonged time
Regardless of the cause, CS is indicated by:
• Hypovolemia (decreased circulating blood volume)
• Hyperkalemia (increased serum potassium)
• Rhabdomyolysis (myoglobulin release from skeletal muscle into the bloodstream)
• Acute tubular necrosis (ATN) resulting from hypovolemia and rhabdomyolysis
Management focuses on preventing (1) ATN from myoglobin release and (2) cardiac dysrhythmias related to hyperkalemia. Kayexalate may reduce serum potassium adequately, but hemodialysis may be required if potassium levels remain high or kidney failure occurs.
Hypovolemic Shock.
Bone is very vascular. Therefore there is a risk for bleeding with bone injury. In addition, trauma can cut nearby arteries and cause hemorrhage, resulting in rapidly developing hypovolemic shock. (The pathophysiology of hypovolemic shock is described in Chapter 39.)
Fat Embolism Syndrome.
Fat embolism syndrome (FES) is another serious complication in which fat globules are released from the yellow bone marrow into the bloodstream within 12 to 48 hours after an injury or other illness. These globules clog small blood vessels that supply vital organs, most commonly the lungs, and impair organ perfusion. FES usually results from long bone fractures or fracture repair but occasionally is seen in patients who have a total joint replacement. It may also occur, although less often, in those with pancreatitis, osteomyelitis, blunt trauma, or sickle cell disease.
The problem can occur at any age or in either gender, but young men between ages 20 and 40 years and older adults between ages 70 and 80 years are at the greatest risk. Patients with fractured hips have the highest risk, but FES is also common in those with fractures of the pelvis.
The earliest manifestation of FES is altered mental status, which is caused by a low arterial oxygen level. Dyspnea and chest pain may follow. Petechiae, a macular, measles-like rash, may appear over the neck, upper arms, or chest and abdomen. This rash is a classic manifestation but can be a late sign.
Abnormal laboratory findings include:
• Increased erythrocyte sedimentation rate (ESR)
• Decreased serum calcium levels
• Decreased red blood cell and platelet counts
These changes in blood values are poorly understood, but they aid in diagnosis of the condition.
FES can result in respiratory failure or death, often from pulmonary edema. When the lungs are affected, the complication may be misdiagnosed as a pulmonary embolism from a blood clot (Chart 54-2).
Venous Thromboembolism.
Venous thromboembolism (VTE) includes deep vein thrombosis (DVT) and its major complication, pulmonary embolism (PE). It is the most common complication of lower extremity surgery or trauma and the most often fatal complication of musculoskeletal surgery. Factors that make patients with fractures most likely to develop VTE include:
• Surgical procedure longer than 30 minutes
• Obesity
• Oral contraceptives or hormones
• History of VTE complications
The pathophysiology and management of VTE is described in Chapter 38.
Infection.
Whenever there is trauma to tissues, the body’s defense system is disrupted. Wound infections are the most common type of infection resulting from orthopedic trauma. They range from superficial skin infections to deep wound abscesses. Infection can also be caused by implanted hardware used to repair a fracture surgically, such as pins, plates, or rods. Clostridial infections can result in gas gangrene or tetanus and can prevent the bone from healing properly.
Bone infection, or osteomyelitis, is most common with open fractures in which skin integrity is lost and after surgical repair of a fracture. For patients experiencing this type of trauma, the risk for hospital-acquired infections is increased. These infections are common, and many are from multidrug-resistant organisms, such as methicillin-resistant Staphylococcus aureus (MRSA). Reducing MRSA infections is a primary desired outcome for all health care agencies.
Chronic Complications.
Ischemic necrosis and delayed bone healing are later complications of musculoskeletal trauma. Ischemic necrosis is sometimes referred to as aseptic or avascular necrosis (AVN) or osteonecrosis. Blood supply to the bone is disrupted, leading to the death of bone tissue. This problem is most often a complication of hip fractures or any fracture in which there is displacement of bone. Surgical repair of fractures also can cause necrosis because the hardware can interfere with circulation. Patients on long-term corticosteroid therapy, such as prednisone, are also at high risk for ischemic necrosis.
Delayed union is a fracture that has not healed within 6 months of injury. Some fractures never achieve union; that is, they never completely heal (nonunion). Others heal incorrectly (malunion). These problems are most common in patients with tibial fractures, fractures that involve many treatment techniques (e.g., cast, traction), and pathologic fractures. Union may also be delayed or not achieved in the older patient. If bone does not heal, he or she typically has chronic pain and immobility from deformity.
Etiology and Genetic Risk
The primary cause of a fracture is trauma from a motor vehicle crash or fall, especially in older adults. The trauma may be a direct blow to the bone or an indirect force from muscle contractions or pulling forces on the bone. Sports, vigorous exercise, and malnutrition are contributing factors. Bone diseases, such as osteoporosis, increase the risk for a fracture in older adults (see Chapter 53). Genetic factors that increase risk for fracture are discussed with these specific health problems throughout this text.
Incidence/Prevalence
The incidence of fractures depends on the location of the injury. Rib fractures are the most common type in the adult population. Femoral shaft fractures occur most often in young and middle-aged adults. The incidence of proximal femur (hip) fractures is highest in older adults. Humeral fractures are common in adults; the older the person, usually the more proximal is the fracture. Wrist (Colles’) fractures are typically seen in middle and late adulthood and usually result from a fall.
Health Promotion and Maintenance
Airbags and seat belts have decreased the number of severe injuries and deaths, but they have increased the number of leg and ankle fractures, especially in older adults. Encourage people to use seat belts, and support legislation for improved vehicle design and re-evaluation of the federal standards for motor vehicle safety. Health teaching should also focus on other risks for musculoskeletal injury, including:
• Osteoporosis screening and education
• Home safety assessment and modification, if needed
• Dangers of drinking and driving
• Drug safety (prescribed, over-the-counter, and illicit)
• Helmet use when riding bicycles, motorcycles, all-terrain vehicles (ATVs), and skateboards
These educational interventions are discussed throughout this book and in other texts. Fall prevention is discussed in detail in Chapter 3 as part of care for older adults.
Patient-Centered Collaborative Care
Assessment
History
If the patient is in severe pain, delay the interview until he or she is more comfortable. Then, ask about the cause of the fracture, which helps in developing an individualized plan of care. Some type of force, such as incisional, crush, acceleration or deceleration, and shearing and friction, leads to most musculoskeletal injuries. As a result, several body systems are often affected.
Incisional injuries, as from a knife wound, and crush injuries cause hemorrhage and decrease blood flow to major organs. Acceleration or deceleration injuries cause direct trauma to the spleen, brain, and kidneys when these organs are moved from their fixed locations in the body. Shearing and friction damage the skin and cause a high level of wound contamination.
Asking about the events leading to the injury helps identify which forces have been experienced and therefore which body systems or parts of the body to assess. For example, a forward fall often results in Colles’ fracture of the wrist because the person tries to catch himself or herself with an outstretched hand. Knowing the mechanism of injury also helps determine whether other types of injury, such as head and spinal cord injury, might be present.
A drug history, including substance abuse, is important regardless of the patient’s age. For example, a young adult may have had an excessive amount of alcohol, which contributed to a motor vehicle crash or to a fall at the work site. Many older adults also consume alcohol and an assortment of prescribed and over-the-counter drugs, which can cause dizziness and loss of balance.
A medical history may identify possible causes of the fracture and gives clues as to how long it will take for the bone to heal. Certain diseases such as bone cancer and Paget’s disease cause pathologic fractures that often do not achieve total healing or union.
Ask about the patient’s occupation and recreational activities. Some occupations are more hazardous than others. For instance, construction work is potentially more physically dangerous than office work. Certain hobbies and recreational activities are also extremely hazardous, such as skiing. Contact sports, such as football and ice hockey, often result in musculoskeletal injuries, including fractures. Other activities do not have such an obvious potential for injury but can cause fractures nonetheless. For instance, daily jogging or running can lead to fatigue fractures.
Physical Assessment/Clinical Manifestations
The patient with a fracture often has trauma to other body systems. Therefore assess all major body systems first for life-threatening complications, including head, chest, and abdominal trauma. For example, some fractures can cause internal organ damage resulting in hemorrhage. When a pelvic fracture is suspected, assess vital signs, skin color, and level of consciousness for indications of possible hypovolemic shock. Check the urine for blood, which indicates possible damage to the urinary system, often the bladder. If the patient cannot void, suspect that the bladder or urethra has been damaged. Complete assessment of these areas is described elsewhere in this text.
For fractures of the shoulder and upper arm, the physical assessment is best done with the patient in a sitting or standing position, if possible, so that shoulder drooping or other abnormal positioning can be seen. Support the affected arm and flex the elbow to promote comfort during the assessment. For more distal areas of the arm, perform the assessment with the patient in a supine position so that the extremity can be elevated to reduce swelling.
Place the patient in a supine position for assessment of the legs and pelvis. A patient with an impacted hip fracture may be able to walk for a short time after injury, although this is not recommended.
When inspecting the site of a possible fracture, look for a change in bone alignment. The bone may appear deformed, a limb may be internally or externally rotated, and/or one or more bones may also be dislocated (out of their joint capsules). Observe for extremity shortening or a change in bone shape.
If the skin is intact (closed fracture), the area over the fracture may be ecchymotic (bruised) from bleeding into the underlying soft tissues. Subcutaneous emphysema, the appearance of bubbles under the skin because of air trapping, may be present but is usually seen later.
Swelling at the fracture site is rapid and can result in marked neurovascular compromise. Gently perform a thorough neurovascular assessment, and compare extremities. Assess skin color and temperature, sensation, mobility, pain, and pulses distal to the fracture site. If the fracture involves an extremity and the patient is not in severe pain, check the nails for capillary refill by applying pressure to the nail and observing for the speed of blood return. If nails are brittle or thick, assess the skin next to the nail. Checking for capillary refill is not as reliable as other indicators of perfusion. Chart 54-3 describes the procedure for a neurovascular assessment, which evaluates circulation, movement, and sensation (CMS function).
Psychosocial Assessment
The psychosocial status of a patient with a fracture depends on the extent of the injury, possible complications, coping ability, and the availability of support systems. Hospitalization is not required for a single, uncomplicated fracture, and the patient returns to usual daily activities within a few days. Examples include a single fracture of a finger, wrist, foot, or toe.
In contrast, a patient suffering severe or multiple traumas may be hospitalized for weeks and may undergo many surgical procedures, treatments, and prolonged rehabilitation. These disruptions in lifestyle can create a high level of stress.
The stresses that result from a long-term condition affect relationships between the patient and family members or friends. Assess the patient’s feelings, and ask how he or she coped with previously experienced stressful events. Body image and sexuality may be altered by deformity, treatment modalities for fracture repair, or long-term immobilization. Assess the availability of support systems, such as family, church, or community groups who can help patients during the acute and rehabilitation phases needed when multiple or severe fractures occur. Active patients of any age or those who are older and live alone may become depressed during the healing process. Acute and chronic pain can decrease energy levels and may also cause sadness or depression.
Laboratory Assessment
No special laboratory tests are available for assessment of fractures. Hemoglobin and hematocrit levels may often be low because of bleeding caused by the injury. If extensive soft-tissue damage is present, the erythrocyte sedimentation rate (ESR) may be elevated, which indicates the expected inflammatory response. If this value increases during fracture healing, the patient may have a bone infection. During the healing stages, serum calcium and phosphorus levels are often increased as the bone releases these elements into the blood.
Imaging Assessment
The health care provider requests standard x-rays and tomograms to confirm a diagnosis of fracture. These reveal the bone disruption, malalignment, or deformity. If the x-ray does not show a fracture but the patient is symptomatic, the x-ray is usually repeated with additional views.
The computed tomography (CT) scan is useful in detecting fractures of complex structures, such as the hip and pelvis. It also identifies compression fractures of the spine. Magnetic resonance imaging (MRI) is useful in determining the amount of soft-tissue damage that may have occurred with the fracture.
Analysis
The most common problems for patients with fractures are:
1 Acute Pain related to one or more fractures, soft-tissue damage, muscle spasm, and edema
2 Potential for neurovascular compromise related to tissue edema and/or bleeding
3 Potential for infection related to a wound caused by an open fracture
4 Impaired Physical Mobility related to acute or chronic pain
Planning and Implementation
Managing Acute Pain
Planning: Expected Outcomes.
The patient with a fracture is expected to state that he or she has adequate pain control after fracture reduction and immobilization.
Interventions.
A fracture can happen anywhere and may be accompanied by multiple injuries to vital organs. Patient-centered collaborative care depends on the severity and extent of the injury and the number of fractures the patient has.
Emergency Care: Fracture.
For any patient who experiences trauma in the community, first call 911 and assess for airway, breathing, and circulation (ABCs, or primary survey). Then provide lifesaving care if needed before being concerned about the fracture (Chart 54-4). If CPR is needed, ensure circulation first, followed by airway and breathing (see Chapter 36).
If the person is clothed, cut away clothing from the fracture site, and remove any jewelry from the affected extremity. Control any bleeding by direct pressure on the area and digital pressure over the artery above the fracture. To prevent shock, place the patient in a supine position and keep him or her warm.
After a head-to-toe assessment (secondary survey) and patient stabilization by the prehospital team, pain is managed with IV opioids such as fentanyl. Cardiac monitoring for patients who are older than 50 years is established before drug administration. To prevent further tissue damage, reduce pain, and increase circulation, the prehospital or emergency team immobilizes the fracture by splinting. An air splint or any object or device that extends to the joints above and below the fracture to immobilize it can be used as a splint. Sterile gauze is placed loosely over open areas to prevent further contamination of the wound.
In the emergency department (ED), physician’s office, or urgent care center, fracture management begins with reduction and immobilization of the fracture, while attending to continued pain assessment and management.
Reduction, or realignment of the bone ends for proper healing, is accomplished by a closed method or an open (surgical) procedure. In some cases, dislocated bones are also reduced, such as when the distal tibia and fibula are dislocated with a fractured ankle. Immobilization is achieved by the use of bandages, casts, traction, internal fixation, or external fixation.
The health care provider selects the treatment method based on the type, location, and extent of the fracture. These interventions prevent further injury and reduce pain.
Nonsurgical Management.
Nonsurgical management includes closed reduction and immobilization with a bandage, splint, cast, or traction. For some small, closed bone fractures in the hand or foot, reduction is not required. Immobilization with an orthotic device or special orthopedic shoe or boot may be the only management during the healing process.
For each modality, the primary nursing concern is assessment and prevention of neurovascular dysfunction or compromise. Assess the patient’s neurovascular status every hour for the first 24 hours and every 1 to 4 hours thereafter, depending on the injury (see Chart 54-3). The patient usually reports discomfort that is unrelieved by analgesics if the bandage, splint, or cast is too tight. Elevate the fractured extremity higher than the heart, and apply ice for the first 24 to 48 hours as needed to reduce edema.
Closed Reduction and Immobilization.
Closed reduction is the most common nonsurgical method for managing a simple fracture. While applying a manual pull, or traction, on the bone, the health care provider moves the bone ends so that they realign. Moderate sedation and/or analgesia is often used during this procedure to decrease pain. An x-ray shows that the bone ends are approximated (aligned) before the bone is immobilized.
Bandages and Splints.
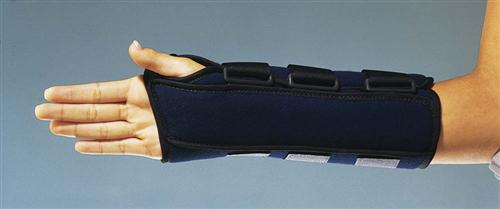
For certain areas of the body, such as the scapula (shoulder) and clavicle (collarbone), an elastic bandage or commercial immobilizer may be used to keep the bone in place during healing. Because upper extremity bones do not bear weight, splints may be sufficient to keep bone fragments in place for a closed fracture. Fig. 54-3 shows a wrist splint for fracture immobilization. Thermoplastic, a durable, flexible material for splinting, allows custom fitting to the patient’s body part. Splints for lower extremities are also custom-fitted using flexible materials and held in place with elastic bandages (e.g., ace wrap).
Casts.
For more complex fractures or fractures of the lower extremity, the physician or orthopedic technician applies a cast to hold bone fragments in place after reduction. A cast is a rigid device that immobilizes the affected body part while allowing other body parts to move. It also allows early mobility and reduces pain. Although its most common use is for fractures, a cast may be applied for correction of deformities (e.g., clubfoot) or for prevention of deformities (e.g., those seen in some patients with rheumatoid arthritis).
Several types of materials are used to make casts. The traditional plaster-of-Paris cast is no longer commonly used for management of most fractures. It requires application of a well-fitted stockinette under the material. If the stockinette is too tight, it may impair circulation. If it is too loose, wrinkles can lead to the development of pressure ulcers. Padding is applied over the stockinette, followed by wet plaster rolls wrapped around the extremity or other body part. The cast feels hot because an immediate chemical reaction occurs, but it soon becomes damp and cool. This type of cast takes 24 to 72 hours to dry, depending on the size and location of the cast. A wet cast feels cold, smells musty, and is grayish. The cast is dry when it feels hard and firm, is odorless, and has a shiny white appearance.
If the skin under the cast is open, the health care provider, orthopedic technician, or specially trained nurse cuts a window in the cast so that the wound can be observed and cared for. The piece of cast removed to make the window must be retained and replaced after wound care to prevent localized edema in the area. This is most important when a window is cut from a cast on an extremity. Tape or elastic bandage wrap may be used to keep the “window” in place. A window is also an access for taking pulses, removing wound drains, or preventing abdominal distention when the patient is in a body or spica cast.
If the cast is too tight, it may be cut with a cast cutter to relieve pressure or allow tissue swelling. The health care provider may choose to bivalve the cast (i.e., cut it lengthwise into two equal pieces) if bone healing is almost complete. Either half of the cast can be removed for inspection or for provision of care. The two halves are then held in place by an elastic bandage wrap.
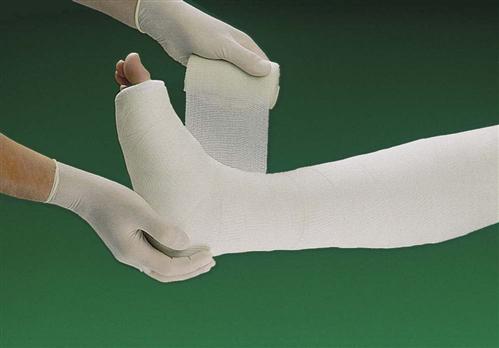
Synthetic materials for casts are much more common and include fiberglass and polyester-cotton knit (Fig. 54-4). These materials are lighter than plaster and require minimal drying time. Fiberglass casts are dry in 10 to 15 minutes and can bear weight 30 minutes after application. Polyester-cotton knit casts take 7 minutes to dry and can withstand weight bearing in about 20 minutes. Some health care providers may use synthetic casts for upper extremities and plaster-of-Paris casts for lower extremities because plaster casts can bear more weight for a longer time. However, newer synthetic materials are stronger than earlier ones. Synthetic casts can be bivalved as needed.
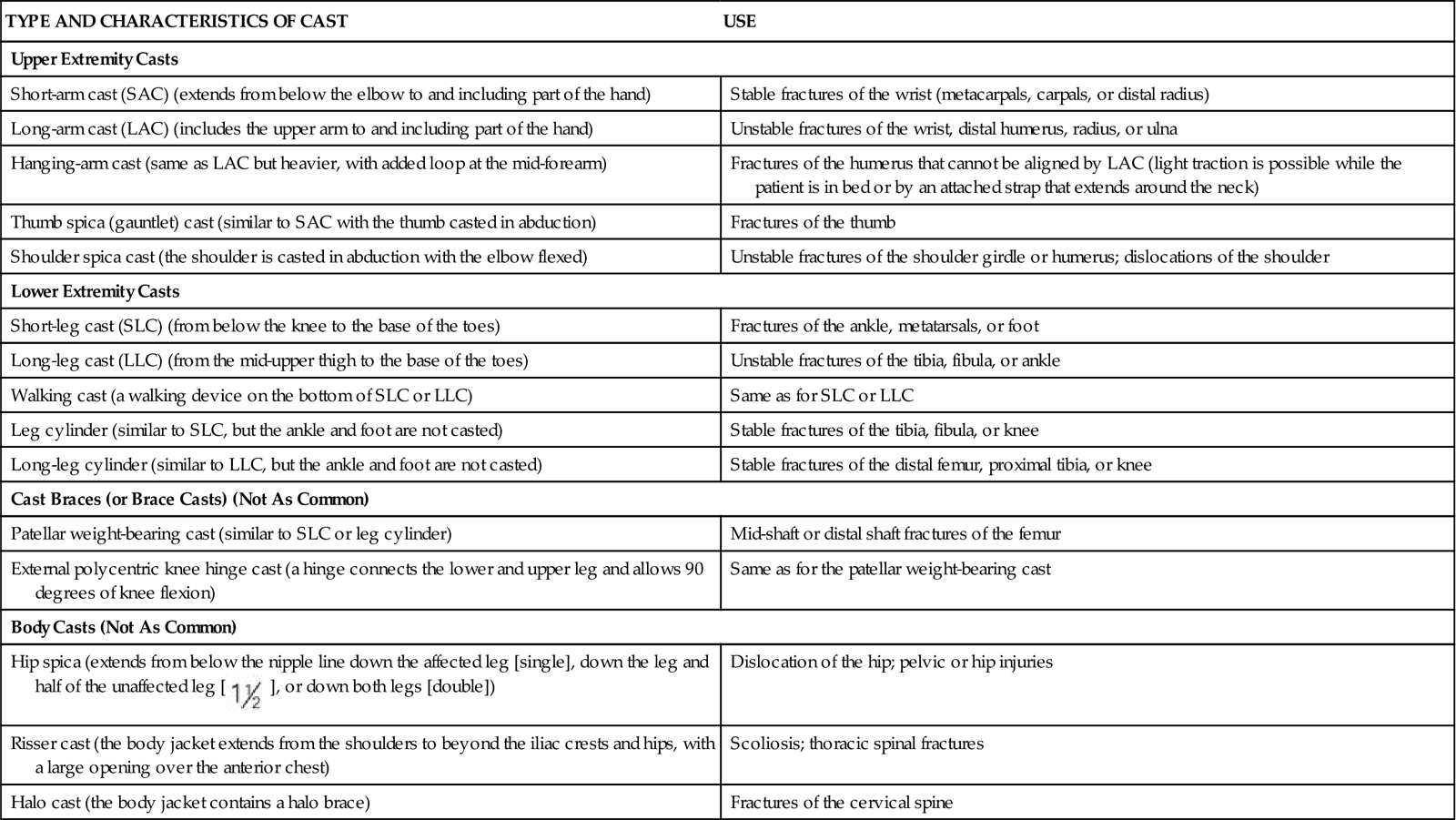
Casts can be generally divided into four main groups: arm casts, leg casts, cast braces, and body or spica casts. Table 54-1 describes specific casts that are used for various parts of the body.
TABLE 54-1
TYPES OF CASTS USED FOR MUSCULOSKELETAL TRAUMA
| TYPE AND CHARACTERISTICS OF CAST | USE |
| Upper Extremity Casts | |
| Short-arm cast (SAC) (extends from below the elbow to and including part of the hand) | Stable fractures of the wrist (metacarpals, carpals, or distal radius) |
| Long-arm cast (LAC) (includes the upper arm to and including part of the hand) | Unstable fractures of the wrist, distal humerus, radius, or ulna |
| Hanging-arm cast (same as LAC but heavier, with added loop at the mid-forearm) | Fractures of the humerus that cannot be aligned by LAC (light traction is possible while the patient is in bed or by an attached strap that extends around the neck) |
| Thumb spica (gauntlet) cast (similar to SAC with the thumb casted in abduction) | Fractures of the thumb |
| Shoulder spica cast (the shoulder is casted in abduction with the elbow flexed) | Unstable fractures of the shoulder girdle or humerus; dislocations of the shoulder |
| Lower Extremity Casts | |
| Short-leg cast (SLC) (from below the knee to the base of the toes) | Fractures of the ankle, metatarsals, or foot |
| Long-leg cast (LLC) (from the mid-upper thigh to the base of the toes) | Unstable fractures of the tibia, fibula, or ankle |
| Walking cast (a walking device on the bottom of SLC or LLC) | Same as for SLC or LLC |
| Leg cylinder (similar to SLC, but the ankle and foot are not casted) | Stable fractures of the tibia, fibula, or knee |
| Long-leg cylinder (similar to LLC, but the ankle and foot are not casted) | Stable fractures of the distal femur, proximal tibia, or knee |
| Cast Braces (or Brace Casts) (Not As Common) | |
| Patellar weight-bearing cast (similar to SLC or leg cylinder) | Mid-shaft or distal shaft fractures of the femur |
| External polycentric knee hinge cast (a hinge connects the lower and upper leg and allows 90 degrees of knee flexion) | Same as for the patellar weight-bearing cast |
| Body Casts (Not As Common) | |
Hip spica (extends from below the nipple line down the affected leg [single], down the leg and half of the unaffected leg [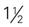 ], or down both legs [double]) ], or down both legs [double]) | Dislocation of the hip; pelvic or hip injuries |
| Risser cast (the body jacket extends from the shoulders to beyond the iliac crests and hips, with a large opening over the anterior chest) | Scoliosis; thoracic spinal fractures |
| Halo cast (the body jacket contains a halo brace) | Fractures of the cervical spine |
When a patient is in bed with an arm cast, teach him or her to elevate the arm above the heart to reduce swelling. The hand should be higher than the heart. Ice may be prescribed for the first 24 to 48 hours. When the patient is out of bed, the arm is supported with a sling placed around the neck to alleviate fatigue caused by the weight of the cast. The sling should distribute the weight over a large area of the shoulders and trunk, not just the neck. Some health care providers prefer that the patient not use a sling after the first few days in an arm cast, particularly a short-arm cast. This encourages normal movement of the mobile joints and enhances bone healing.
A leg cast allows mobility and requires the patient to use ambulatory aids such as crutches. A cast shoe, sandal, or boot that attaches to the foot or a rubber walking pad attached to the sole of the cast assists in ambulation (if weight bearing is allowed) and helps prevent damage to the cast. Teach the patient to elevate the affected leg on several pillows to reduce swelling and to apply ice for the first 24 hours or as prescribed.
A body cast encircles the trunk of the body and is not commonly used for adults. A spica cast encases a portion of the trunk and one or two extremities. A patient with either of these casts presents a special challenge for nursing care. Potential complications related to severe impairment in mobility include:
Cast syndrome (superior mesenteric artery syndrome), an uncommon but serious complication, may be seen in orthopedic patients who have been placed in a hip spica or body cast. Partial or complete upper intestinal obstruction results in classic symptoms: abdominal distention, epigastric pain, nausea, and vomiting. The vomiting often occurs after meals, and patients may have normal bowel sounds. Partial obstruction occurs initially from compression of the third portion of the duodenum between the superior mesenteric artery and the aorta. This can progress to complete obstruction from duodenal edema caused by continued vomiting and distention. Placing a window in the abdominal portion of the cast or bivalving the cast may be sufficient to prevent or relieve pressure on the duodenum. Management of intestinal obstruction is the same as for any patient with this complication.
Before the cast is applied, explain the purpose of the cast and the procedure for its application. With a plaster cast, warn the patient about the heat that will be felt immediately after the wet cast is applied. Do not cover the new cast. Allow for air-drying.
For preventing contamination by urine or feces, the perineal area of a dry long-leg or body cast may be covered in plastic. Fracture pans are preferred over traditional bedpans because they are smaller and more comfortable. Remind UAP to take care to prevent spillage onto the cast.
Once the plaster cast is dry, inspect it at least once every 8 hours for drainage, cracking, crumbling, alignment, and fit. Plaster casts act like sponges and absorb drainage, whereas synthetic casts act like a wick pulling drainage away from the drainage site. Padding can also absorb wound drainage. Document the presence of any drainage on the cast. However, the evidence is not clear on whether drainage should be circled on the cast because it may increase anxiety and is not a reliable indicator of drainage amount. Immediately report to the health care provider any sudden increases in the amount of drainage or change in the integrity of the cast. After swelling decreases, it is not uncommon for the cast to become too loose and need replacement. If the patient is not admitted to the hospital, provide instructions regarding cast care.
During hospitalization, assess for other complications resulting from casting that can be serious and life threatening, such as infection, circulation impairment, and peripheral nerve damage. If the patient returns home after cast application, teach him or her how to monitor for these complications and when to notify the health care provider.
Infection most often results from the breakdown of skin under the cast (pressure necrosis). If pressure necrosis occurs, the patient typically reports a very painful “hot spot” under the cast and the cast may feel warmer in the affected area. Teach the patient or family to smell the area for mustiness or an unpleasant odor that would indicate infected material. If the infection progresses, a fever may develop.
Circulation impairment and peripheral nerve damage can result from tightness of the cast. Teach the patient to assess for circulation at least daily, including the ability to move the area distal to the extremity, numbness, and increased pain.
The patient with a cast may be immobilized for a prolonged period, depending on the extent of the fracture and the type of cast. Assess for complications of immobility, such as skin breakdown, pneumonia, atelectasis, thromboembolism, and constipation. Before the cast is removed, inform the patient that the cast cutter will not injure the skin but that heat may be felt during the procedure.
Because of prolonged immobilization, a joint may become contracted, usually in a fixed state of flexion. Osteoarthritis and osteoporosis may develop from lack of weight bearing. Muscle can also atrophy from lack of exercise during prolonged immobilization of the affected body part, usually an extremity.
Traction.
Traction is the application of a pulling force to a part of the body to provide reduction, alignment, and rest. It is also used as a last resort to decrease muscle spasm (thus relieving pain) and prevent or correct deformity and tissue damage. A patient in traction is often hospitalized, but in some cases, home care is possible even for skeletal traction.
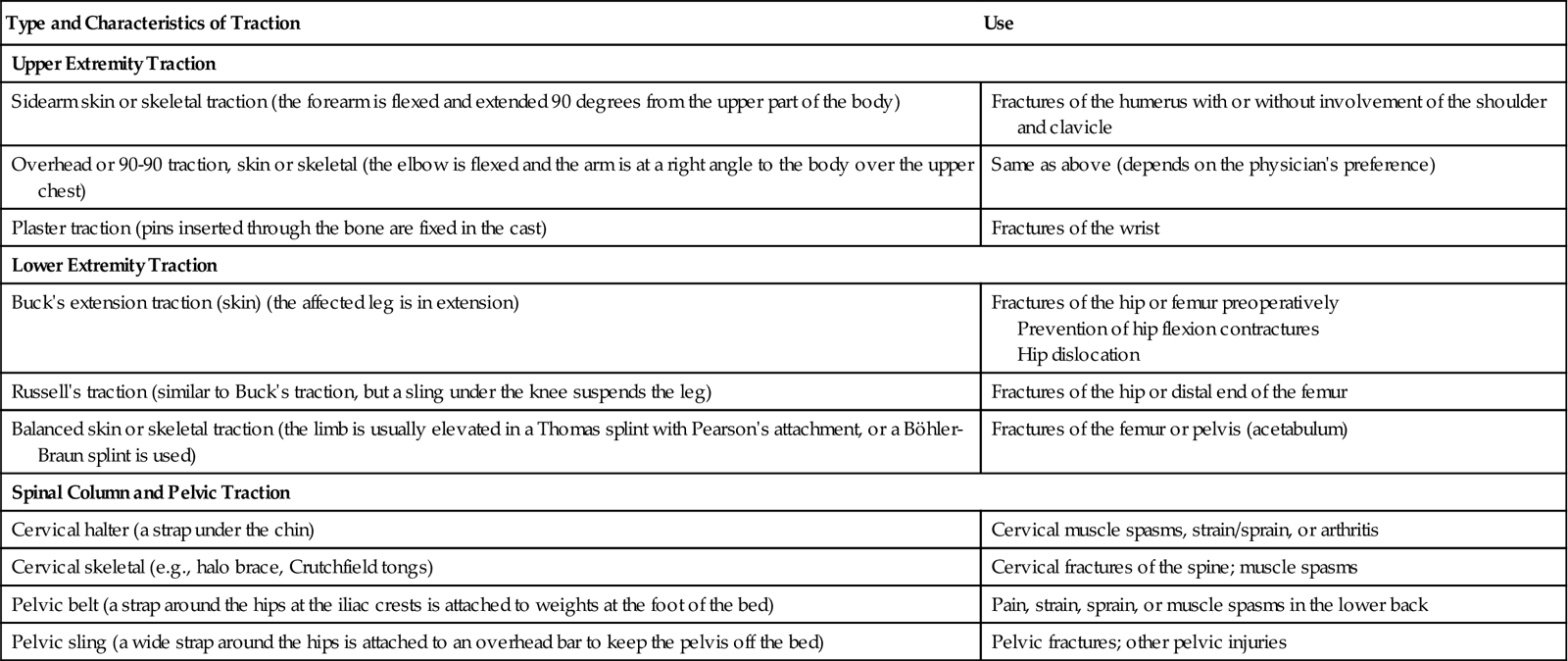
Mechanical traction can be either:
Traction may also be classified as running traction or balanced suspension. In running traction, the pulling force is in one direction and the patient’s body acts as countertraction. Moving the body or bed position can alter the countertraction force. Balanced suspension provides the countertraction so that the pulling force of the traction is not altered when the bed or patient is moved. This allows for increased movement and facilitates care (Table 54-2).
TABLE 54-2
TYPES OF TRACTION USED FOR MUSCULOSKELETAL TRAUMA
| Type and Characteristics of Traction | Use |
| Upper Extremity Traction | |
| Sidearm skin or skeletal traction (the forearm is flexed and extended 90 degrees from the upper part of the body) | Fractures of the humerus with or without involvement of the shoulder and clavicle |
| Overhead or 90-90 traction, skin or skeletal (the elbow is flexed and the arm is at a right angle to the body over the upper chest) | Same as above (depends on the physician’s preference) |
| Plaster traction (pins inserted through the bone are fixed in the cast) | Fractures of the wrist |
| Lower Extremity Traction | |
| Buck’s extension traction (skin) (the affected leg is in extension) | Fractures of the hip or femur preoperatively Prevention of hip flexion contractures Hip dislocation |
| Russell’s traction (similar to Buck’s traction, but a sling under the knee suspends the leg) | Fractures of the hip or distal end of the femur |
| Balanced skin or skeletal traction (the limb is usually elevated in a Thomas splint with Pearson’s attachment, or a Böhler-Braun splint is used) | Fractures of the femur or pelvis (acetabulum) |
| Spinal Column and Pelvic Traction | |
| Cervical halter (a strap under the chin) | Cervical muscle spasms, strain/sprain, or arthritis |
| Cervical skeletal (e.g., halo brace, Crutchfield tongs) | Cervical fractures of the spine; muscle spasms |
| Pelvic belt (a strap around the hips at the iliac crests is attached to weights at the foot of the bed) | Pain, strain, sprain, or muscle spasms in the lower back |
| Pelvic sling (a wide strap around the hips is attached to an overhead bar to keep the pelvis off the bed) | Pelvic fractures; other pelvic injuries |
The two most common types of traction are skin and skeletal traction. Skin traction involves the use of a Velcro boot (Buck’s traction) (Fig. 54-5), belt, or halter, which is usually secured around the affected leg. The primary purpose of skin traction is to decrease painful muscle spasms that accompany hip fractures. The weight is used as a pulling force and is limited to 5 to 10 pounds (2.3 to 4.5 kg) to prevent injury to the skin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree