Margaret Elaine McLeod
Care of Patients with Diabetes Mellitus
Learning Outcomes
Safe and Effective Care Environment
Health Promotion and Maintenance
Psychosocial Integrity
Physiological Integrity
14 Explain the effects of insulin on carbohydrate, protein, and fat metabolism.
15 Explain how to mix different kinds of insulin together.
17 Explain how to perform foot assessment and foot care for the patient with diabetes.
![]()
http://evolve.elsevier.com/Iggy/
Animation: Insulin Function
Answer Keys for NCLEX Examination Challenges and Decision-Making Challenges
Audio Glossary
Concept Map Creator
Concept Map: Diabetes Mellitus—Type 2
Key Points
Review Questions for the NCLEX® Examination
Diabetes mellitus (DM) is a chronic metabolic disease that requires lifelong behavioral and lifestyle changes. A collaborative approach helps the patient successfully manage the disease. As part of the team, you will plan, organize, and coordinate care with other health care team members to provide care and education and promote the patient’s health and well-being.
Diabetes is a major public health problem, and its complications, especially hypertension and hyperlipidemia (high blood lipid levels), cause many serious health problems. In the United States, diabetes mellitus (DM) is a leading cause of blindness, end-stage kidney disease, and foot or leg amputations. Many people have undiagnosed diabetes and, among those who are diagnosed, many have continuous high blood glucose levels. The complications of DM can be greatly reduced with glycemic (blood glucose) control along with management of hypertension and hyperlipidemia. Thus nursing priorities focus on helping the patient with diabetes achieve and maintain lifestyle changes that prevent long-term complications by keeping blood glucose levels and cholesterol levels as close to normal as possible (Young, 2011).
Pathophysiology
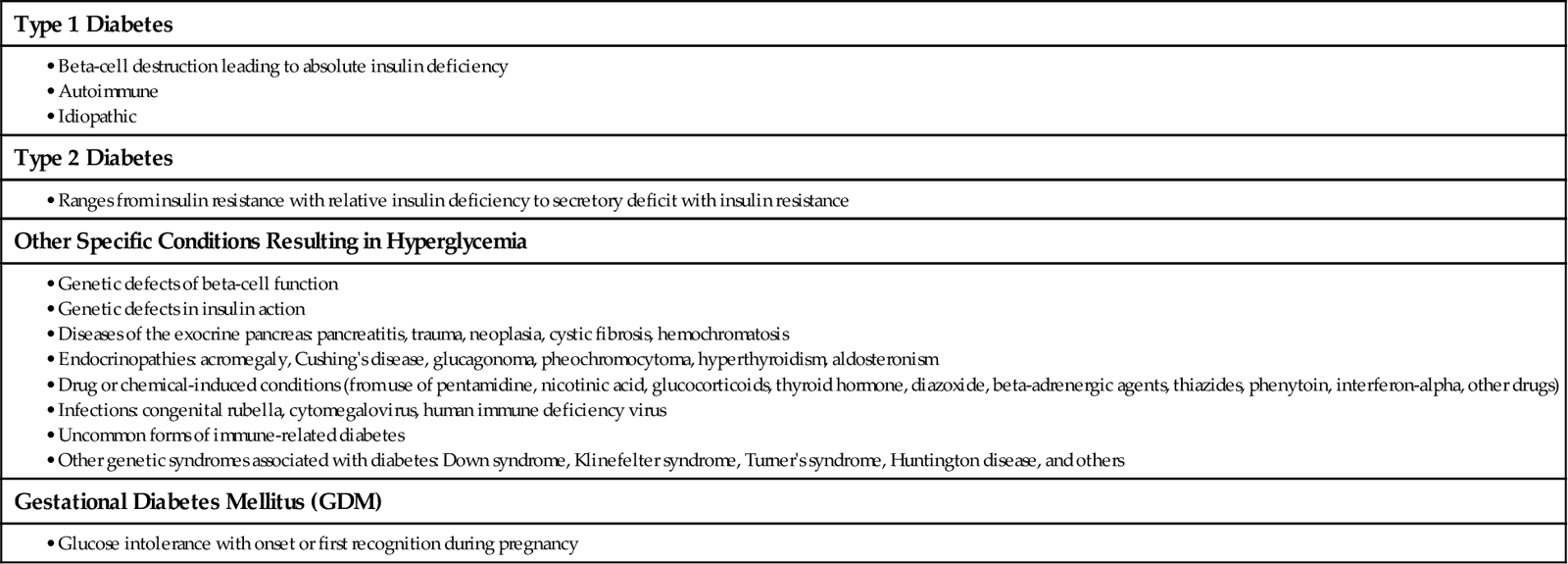
Classification of Diabetes
For all types of diabetes mellitus (DM), the main feature is chronic hyperglycemia (high blood glucose level) resulting from problems with insulin secretion, insulin action, or both. The disease is classified by the underlying problem causing a lack of insulin and the severity of the insulin deficiency. Table 67-1 outlines the types of DM.
TABLE 67-1
CLASSIFICATION OF DIABETES MELLITUS
Data from American Diabetes Association (ADA). (2010a). Position statement: Diagnosis and classification of diabetes mellitus, Diabetes Care, 33(Suppl. 1), 62-69.
The Endocrine Pancreas
The pancreas has mostly exocrine functions that are related to digestion and endocrine functions that are related to blood glucose control. The endocrine portion of the pancreas has about 1 million small glands, the islets of Langerhans, scattered through the organ. The two types of islet cells important to glucose control are the alpha cells, which secrete glucagon, and the beta cells, which produce insulin and amylin. Glucagon is a “counterregulatory” hormone that has actions opposite those of insulin. It prevents hypoglycemia (low blood glucose levels) by triggering the release of glucose from cell storage sites. Insulin prevents hyperglycemia by allowing body cells to take up, use, and store carbohydrate, fat, and protein.
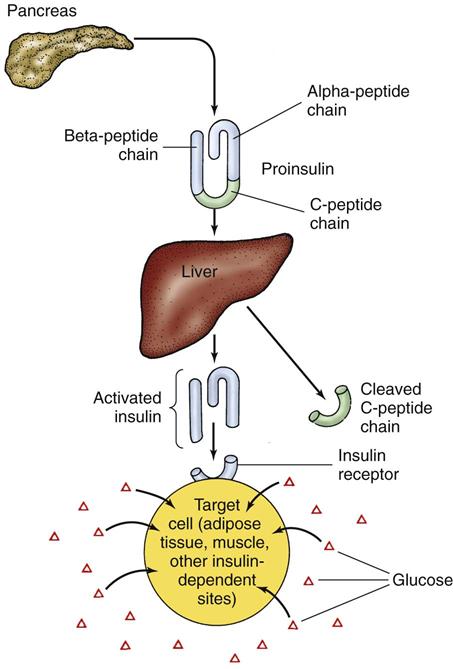
Active insulin is a protein made up of 51 amino acids. It is initially produced as inactive proinsulin, a prohormone that contains an additional amino acid chain (the C-peptide chain). Proinsulin is converted into active insulin by removal of the C-peptide (Fig. 67-1).
About 40 to 50 units of insulin is secreted daily directly into liver circulation in a two-step manner. It is secreted at low levels during fasting (basal insulin secretion) and at increased levels after eating (prandial). An early burst of insulin secretion occurs within 10 minutes of eating. This is followed by an increasing release that lasts until the blood glucose level is normal.
Glucose Homeostasis
Glucose is the main fuel for central nervous system (CNS) cells. Because the brain cannot produce or store much glucose, it needs a continuous supply from circulation to prevent neuronal dysfunction and cell death. Other organs can use both glucose and fatty acids to generate energy. Glucose is stored inside cells as glycogen in the liver and muscles, and free fatty acids are stored as triglyceride in fat cells. Fat is the most efficient means of storing energy. Fat has 9 calories of stored energy per gram. Protein and carbohydrate have only 4 calories per gram. During a prolonged fast or after illness or injury, proteins are broken down and some of the amino acids are converted into glucose.
Several organs and hormones play a role in maintaining glucose homeostasis. During the fasting state, when the stomach is empty, blood glucose is maintained between 60 and 150 mg/dL (3.3 and 8.3 mmol/L) by a balance between glucose uptake by cells and glucose production by the liver. Insulin plays a pivotal role in this process.
Movement of glucose into some cells requires the presence of specific carrier proteins, glucose transport (GLUT) proteins and insulin. Insulin is like a “key” that opens “locked” membranes to glucose, allowing glucose in the blood to move into cells to generate energy. Insulin starts this action by binding to insulin receptors on the cell membranes, which changes membrane permeability to glucose.
Insulin exerts many effects on metabolism and cellular processes in different body tissues and organs. The main metabolic effects of insulin are to stimulate glucose uptake in skeletal muscle and heart muscle and to suppress liver production of glucose and very-low-density lipoprotein (VLDL). In the liver, insulin promotes the production and storage of glycogen (glycogenesis) at the same time that it inhibits glycogen breakdown into glucose (glycogenolysis). It increases protein and lipid (fat) synthesis and inhibits ketogenesis (conversion of fats to acids) and gluconeogenesis (conversion of proteins to glucose). In muscle, insulin promotes protein and glycogen synthesis. In fat cells, it promotes triglyceride storage. Overall, insulin keeps blood glucose levels from becoming too high and helps keep blood lipid levels in the normal range.
In the fasting state (not eating for 8 hours), insulin secretion is suppressed, which leads to increased gluconeogenesis in the liver and kidneys, along with increased glucose generation by the breakdown of liver glycogen. In the fed state, insulin released from pancreatic beta cells reverses this process. Instead, glycogen breakdown and gluconeogenesis are inhibited. At the same time, insulin also enhances glucose uptake and use by cells and reduces both fat breakdown (lipolysis) and protein breakdown (proteolysis). When more glucose is present in liver cells than can be metabolized for energy or stored as glycogen, insulin causes the excess glucose to be converted to free fatty acids (FFAs). These extra FFAs are deposited as fat in fat cells.
Glucose in the blood after a meal is controlled by the emptying rate of the stomach and delivery of nutrients to the small intestine where they are absorbed into circulation. Incretin hormones (e.g., GLP-1), secreted in response to the presence of food in the stomach, have several actions. They increase insulin secretion, inhibit glucagon secretion, and slow the rate of gastric emptying, thereby preventing hyperglycemia after meals.
Counterregulatory hormones increase blood glucose by actions opposite those of insulin when more energy is needed. Glucagon is the main counterregulatory hormone. Other hormones that increase blood glucose levels are epinephrine, norepinephrine, growth hormone, and cortisol. The combined actions of insulin and counterregulatory hormones (discussed in the next section) keep blood glucose levels in the range of 60 to 100 mg/dL (3.3 to 5.6 mmol/L) to support brain functions. When glucose levels fall, insulin secretion stops and glucagon is released. Glucagon causes the release of glucose from the liver. Liver glucose is made through breakdown of glycogen to glucose (glycogenolysis) and conversion of amino acids into glucose (gluconeogenesis). When liver glucose is unavailable, the breakdown of fat (lipolysis) and the breakdown of proteins (proteolysis) provide fuel for energy.
Absence of Insulin
Insulin is needed to move glucose into most body tissues. The lack of insulin in diabetes, from either a lack of production or a problem with insulin use at its cell receptor, prevents some cells from using glucose for energy. The body then breaks down fat and protein in an attempt to provide energy and also increases the levels of counterregulatory hormones in an attempt to make glucose from other sources. Table 67-2 outlines the body’s response to insufficient insulin.
TABLE 67-2
PHYSIOLOGIC RESPONSE TO INSUFFICIENT INSULIN
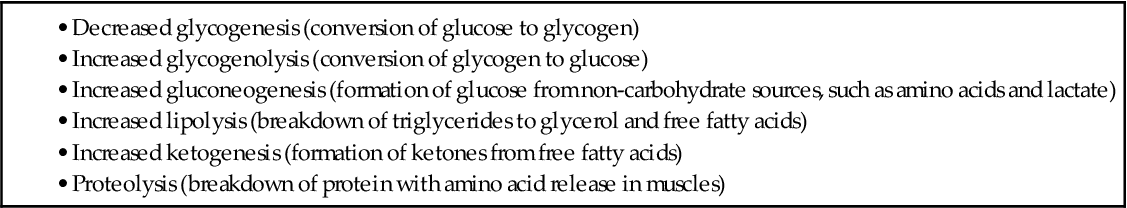
Without insulin, glucose builds up in the blood, causing hyperglycemia, which is high blood glucose levels. Hyperglycemia causes fluid and electrolyte imbalances, leading to the classic symptoms of diabetes: polyuria, polydipsia, and polyphagia.
Polyuria is frequent and excessive urination and results from an osmotic diuresis caused by excess glucose in the urine. As a result of diuresis, sodium, chloride, and potassium are excreted in the urine and water loss is severe. Dehydration results, and polydipsia (excessive thirst) occurs. Because the cells receive no glucose, cell starvation triggers polyphagia (excessive eating). Despite eating vast amounts of food, the person remains in starvation until insulin is available to move glucose into the cells.
With insulin deficiency, fats break down, releasing free fatty acids. Conversion of fatty acids to ketone bodies (small acids) provides a backup energy source. Because ketone bodies, or “ketones,” are abnormal breakdown products of fatty acids, they collect in the blood when insulin is not available, leading to metabolic acidosis.
The dehydration that occurs with diabetes leads to hemoconcentration (an increased blood concentration), hypovolemia (a decreased blood volume), hyperviscosity (thick, concentrated blood), poor tissue perfusion, and hypoxia (poor tissue oxygenation), especially to the brain. Hypoxic cells do not metabolize glucose efficiently, the Krebs’ cycle is blocked, and lactic acid increases, causing more acidosis.
The excess acids caused by absence of insulin increase hydrogen ion (H+) and carbon dioxide (CO2) levels in the blood, causing metabolic acidosis. These products trigger the respiratory centers of the brain to increase the rate and depth of respiration in an attempt to excrete more carbon dioxide and acid. This type of breathing is known as Kussmaul respiration. Acetone is exhaled, giving the breath a “fruity” odor. When the lungs can no longer offset acidosis, the blood pH drops. Arterial blood gas studies show a metabolic acidosis (decreased pH with decreased arterial bicarbonate [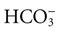 ] levels) and compensatory respiratory alkalosis (decreased partial pressure of arterial carbon dioxide [PaCO2]).
] levels) and compensatory respiratory alkalosis (decreased partial pressure of arterial carbon dioxide [PaCO2]).
Insulin lack initially causes potassium depletion. With the increased fluid loss from hyperglycemia, excessive potassium is excreted in the urine, leading to low serum potassium levels. High serum potassium levels may occur in acidosis because of the shift of potassium from inside the cells to the blood. Serum potassium levels in DM, then, may be low (hypokalemia), high (hyperkalemia), or normal, depending on hydration, the severity of acidosis, and the patient’s response to treatment. Chapter 14 discusses acid-base balance and acidosis in more detail.
Acute Complications of Diabetes
Three glucose-related emergencies can occur in patients with diabetes:
• Diabetic ketoacidosis (DKA) caused by lack of insulin and ketosis
• Hyperglycemic-hyperosmolar state (HHS) caused by insulin deficiency and profound dehydration
All three problems require emergency treatment and can be fatal if treatment is delayed or incorrect. These problems and their interventions are described later.
Chronic Complications of Diabetes
Diabetes mellitus (DM) can lead to health problems and early death because of changes in large blood vessels (macrovascular) and small blood vessels (microvascular) in tissues and organs. Complications result from poor tissue circulation and cell death. Macrovascular complications, including coronary heart disease, cerebrovascular disease, and peripheral vascular disease, lead to increased early death. Microvascular complications of blood vessel structure and function lead to nephropathy (kidney dysfunction), neuropathy (nerve dysfunction), and retinopathy (vision problems). Explanations for these diabetic vascular complications include:
• Chronic hyperglycemia thickens basement membranes, which causes organ damage.
• Glucose toxicity directly or indirectly affects functional cell integrity.
• Chronic ischemia in small blood vessels causes connective tissue hypoxia and microischemia.
Chronic high blood glucose levels are the main cause of microvascular complications and allow premature development of macrovascular complications. Additional risk factors that contribute to poor health outcomes for people with DM include smoking, physical inactivity, increased body weight, hypertension, and excessive blood levels of cholesterol and other fats. Many of these factors can be modified to reduced complications related to DM.
The Diabetes Control and Complications Trial (DCCT) showed that hyperglycemia is a critical factor for long-term complications in patients with type 1 DM. Intensive therapy aiming for blood glucose levels as close to normal as possible delays the onset and progression of retinopathy, nephropathy, neuropathy, and macrovascular disease. Additional studies show that intensive therapy with lowered blood glucose levels delays the onset of retinopathy, nephropathy, and neuropathy in patients with type 2 DM. A strong relationship exists between microvascular complications and blood glucose levels. For every percentage point decrease in HbA1c (hemoglobin A1c), a 35% reduction in the risk for kidney and eye complications has been shown.
Macrovascular Complications
Cardiovascular Disease.
Diabetes mellitus (DM) is associated with a reduced life span, largely as a result of cardiovascular disease (CVD). Most patients with DM die as a result of a thrombotic event, usually myocardial infarction (MI). DM also affects the heart muscle, causing both systolic and diastolic heart failure. Left ventricular dysfunction with heart failure and fatal cardiac dysrhythmias are more common after MI in patients with DM.
Patients with diabetes, those with prediabetes, and those with metabolic syndrome are at increased risk for CVD. This excess risk affects women to a greater degree than men and is influenced by the patient’s ethnic group. The Adult Treatment Panel III of the National Cholesterol Education Program recommends that diabetes be considered a “coronary heart disease risk equivalent” and a target for aggressive reduction of risk factors.
Patients with DM often also have the traditional cardiovascular risk factors of obesity, hypertension, dyslipidemia, and sedentary lifestyle. Cigarette smoking and a positive family history greatly increase risk for CVD. Kidney disease, indicated by albuminuria (presence of albumin in the urine), increases the risk for coronary heart disease and mortality from MI. Patients with DM often have higher levels of C-reactive protein (CRP), an acute-phase inflammatory marker associated with increased risk for cardiovascular problems and death.
Cardiovascular complication rates can be reduced through aggressive management of hyperglycemia, hypertension, and hyperlipidemia. The American Diabetes Association (ADA) recommends that blood pressure be maintained below 130/80 mm Hg and that low-density lipoprotein (LDL) cholesterol remain below 100 mg/dL (2.60 mmol/L) for patients without manifestations of CVD and to less than 70 mg/dL (1.8 mmol/L) for patients with manifestations of CVD (ADA, 2010b). Diets high in saturated fat raise total cholesterol and LDL cholesterol levels, which increase the risk for coronary artery disease. Lifestyle modifications that focus on reducing saturated fat, trans fat, and cholesterol intake; increasing intake of omega-3 fatty acids, fiber, and plant sterols; weight loss (if indicated); and increasing physical activity are recommended to improve the lipid profile for patients with DM (ADA, 2010b).
Priority nursing actions focus on interventions to reduce modifiable risk factors associated with CVD, such as smoking cessation, diet, exercise, blood pressure control, maintenance of prescribed aspirin use, and maintenance of prescribed lipid-lowering drug therapy.
Cerebrovascular Disease.
The risk for stroke is 2 to 4 times higher in people with DM compared with those who do not have the disease. Diabetes also increases the likelihood of severe carotid atherosclerosis. Hypertension, hyperlipidemia, nephropathy, peripheral vascular disease, and alcohol and tobacco use further increase the risk for stroke in people with DM.
DM affects stroke outcomes as well. Patients with DM are likely to suffer irreversible brain damage with carotid emboli that produce only transient ischemic attacks in people without DM. Elevated blood glucose levels at the time of the stroke may lead to greater brain injury and higher mortality.
In addition, chronic hyperglycemia with microvascular disease may contribute to neuronal damage, brain atrophy, and cognitive impairment. These problems are more frequent and more severe in patients who have longer-duration DM and an increase in the complications of neuropathy and retinopathy (Roberts et al., 2008).
Microvascular Complications
Eye and Vision Complications.
Legal blindness (a corrected visual acuity of 20/200 or less) is 25 times more common in patients with diabetes. Diabetic retinopathy (DR) is strongly related to the duration of diabetes. After 20 years of DM, nearly all patients with type 1 disease and most with type 2 disease have some degree of retinopathy. Unfortunately, DR has few manifestations until vision loss occurs.
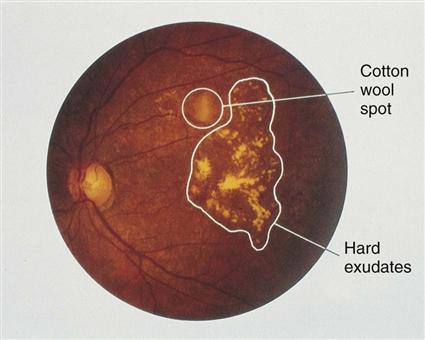
The cause and progression of DR are related to problems that block retinal blood vessels and cause them to leak, leading to retinal hypoxia. Nonproliferative diabetic retinopathy (Fig. 67-2) causes structural problems in retinal vessels, including areas of poor retinal circulation, edema, hard fatty deposits in the eye, and retinal hemorrhages. Microaneurysms are small capillary wall dilations in retinal vessels that form throughout the eye and leak fluid and blood into the retina. This leakage causes retinal edema and hard exudates.
Other retinal problems include retinal hemorrhages, optic nerve atrophy from hypoxia, and venous beading. Venous beading is the abnormal appearance of retinal veins in which areas of swelling and constriction along a segment of vein resemble links of sausage. It occurs in areas of retinal ischemia. Nonproliferative diabetic retinopathies develop slowly and rarely cause reduced vision to the point of blindness.
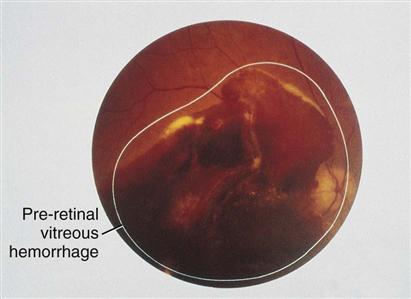
Proliferative diabetic retinopathy is the growth of new retinal blood vessels, also known as neovascularization. When retinal blood flow is poor and hypoxia develops, retinal cells secrete growth factors that stimulate formation of new blood vessels in the eye. These new vessels are thin, fragile, and bleed easily, leading to eye hemorrhage and vision loss (Fig. 67-3).
Vision loss from DR has several mechanisms. Central vision may be impaired by macular edema, which can occur at any stage of DR. Diabetic macular edema is characterized by increased blood vessel permeability and deposits of hard exudates at the center of the retina. This problem is the main cause of vision loss in the person with DM. Vision loss also occurs from macular degeneration, corneal scarring, and changes in lens shape or clarity.
Hyperglycemia may cause blurred vision, even with eyeglasses. Hypoglycemia may cause double vision. Cataracts occur at a younger age and progress faster among patients with DM. Open-angle glaucoma also is more common in patients with DM. The management of cataracts and glaucoma is the same as for patients who do not have diabetes (see Chapter 49).
Control of blood glucose, blood pressure, and blood lipid level is important in preventing DR. Thus patients with DM should have routine ophthalmic evaluations to detect vision problems early before vision loss occurs.
Diabetic Neuropathy.
Neuropathy is a progressive deterioration of nerves that results in loss of nerve function. It is a common complication of DM and often involves all parts of the body. Damage to sensory nerve fibers results in either pain or loss of sensation. Damage to motor nerve fibers results in muscle weakness. Damage to nerve fibers in the autonomic nervous system can cause dysfunction in every part of the body. This combination of factors leads to the nerve damage in diabetic neuropathy (Bedlack, 2009):
• Metabolic factors of hyperglycemia, long duration of DM, hyperlipidemia, low insulin levels
• Damaged blood vessels leading to reduced neuronal oxygen and other nutrients
• Autoimmune neuronal inflammation
• Increased genetic susceptibility to nerve damage
Hyperglycemia leads to neuropathy through blood vessel changes that cause nerve hypoxia. Both the axon and its myelin sheath are damaged by reduced blood flow, resulting in blocked nerve impulse transmission. Excessive glucose is converted to sorbitol, which collects in nerves. The increased sorbitol also impairs motor nerve conduction. Common diabetic neuropathies are listed in Table 67-3. Autonomic nervous system neuropathy leads to problems in cardiovascular, GI, and urinary function. Keeping blood glucose levels in the normal range can slow the development and progression of diabetic neuropathies.
TABLE 67-3
FEATURES OF DIABETIC NEUROPATHY
| COMPLICATION | MANIFESTATION | |
| Diffuse Neuropathies | ||
| Distal symmetric polyneuropathy | Sensory alterations | Paresthesias: burning/tingling sensations, starting in toes and moving up legs |
| Dysesthesias: burning, stinging, or stabbing pain | ||
| Anesthesia: loss of sensation | ||
| Motor alterations in intrinsic muscles of foot | Foot deformities: high arch, claw toes, hammertoes; shift of weight-bearing to metatarsal heads and tips of toes | |
| Autonomic neuropathy | Anhidrosis | Drying, cracking of skin |
| Gastrointestinal | Delayed gastric emptying, gastric retention, early satiety, bloating, nausea, vomiting, anorexia, constipation, diarrhea | |
| Neurogenic bladder | Atonic bladder, urinary retention | |
| Impotence | Erectile dysfunction | |
| Cardiovascular autonomic neuropathy | Early fatigue, weakness with exercise, orthostatic hypotension | |
| Defective counterregulation | Loss of warning signs of hypoglycemia | |
| Focal Neuropathies | ||
| Focal ischemia | Thoracolumbar radiculopathy with sensory and reflex loss | Pain radiating across back, side, and front of chest or abdomen |
| Cranial nerve palsies, third and sixth nerves | Sudden diplopia or ptosis; eye pain | |
| Amyotrophy | Pain; asymmetric weakness; wasting of iliopsoas, quadriceps, and adductor muscles | |
| Entrapment neuropathies | Median nerve | Carpal tunnel syndrome |
| Popliteal nerve/knee | Footdrop | |
| Posterior tibial nerve at tarsal tunnel | Tarsal tunnel syndrome: sensory impairment in sole of foot; weakness of intrinsic muscles of foot; burning pain and paresthesias at ankle and plantar surface | |
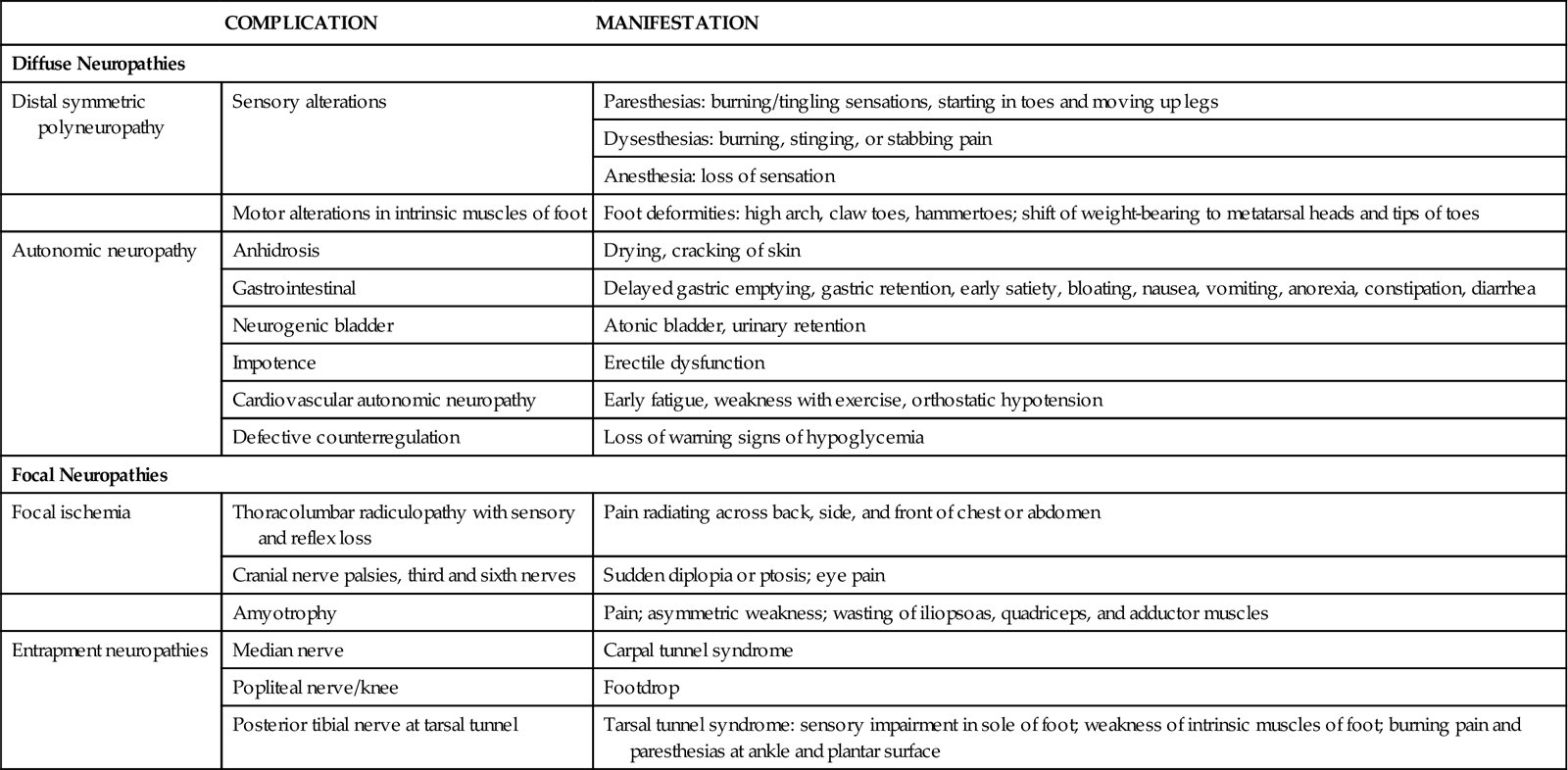
Diabetic neuropathy can be focal or diffuse, each with different causes and rates of progression. Diffuse neuropathies are the most common neuropathies in DM and involve widespread nerve function loss. They have a slow onset, affect both sides of the body, involve motor and sensory nerves, progress slowly, are permanent, and include autonomic nerve dysfunction. Late complications include foot ulcers and deformities.
Focal neuropathies affect a single nerve or nerve group and usually are caused by an acute ischemic event or by nerve trapping. Both problems lead to nerve damage or nerve death. Ischemic neuropathies occur when the blood supply to a nerve or nerve group is disrupted. The symptoms begin suddenly, affect only one side of the body area, and are self-limiting. Recovery time varies. The most common neuropathies affect the nerves that control the eye muscles. Manifestations begin with pain on one side of the face near the affected eye. The eye muscles become paralyzed, resulting in double vision. The problem usually resolves in 2 to 3 months.
Entrapment neuropathies stem from trapping and compressing a nerve in a body compartment or between tissues. Symptoms begin gradually and can occur anywhere. They may be bilateral, having a waxing and waning course without spontaneous recovery. An example of focal entrapment neuropathy is carpal tunnel syndrome.
Cardiovascular autonomic neuropathy (CAN) affects sympathetic and parasympathetic nerves of the heart and blood vessels. This problem contributes to left ventricular dysfunction, painless myocardial infarctions, and exercise intolerance. Most often, CAN leads to orthostatic (postural) hypotension and syncope (brief loss of consciousness on standing). These problems are due to failure of the heart and arteries to adjust to position changes by increasing heart rate and vascular tone. As a result, blood flow to the brain is interrupted briefly. Orthostatic hypotension and syncope increase the risk for falls, especially among older adults.
Autonomic neuropathy can affect the entire GI system. Common GI problems from diabetic neuropathy include gastroesophageal reflex, delayed gastric emptying and gastric retention, early satiety, heartburn, nausea, vomiting, and anorexia. Sluggish movement of the small intestine can lead to bacterial overgrowth, which causes bloating, gas, and diarrhea. Diarrhea caused by diabetes is chronic, may be severe, and often occurs at night. Constipation, the most common GI problem with DM, is intermittent and may alternate with bouts of diarrhea. Gastroparesis (delay in gastric emptying) is a cause of hypoglycemia.
Urinary problems from neuropathy results in incomplete emptying and urine retention, which leads to urinary infection and kidney problems. Manifestations include frequency, urgency, and incontinence.
Diabetic Nephropathy.
Nephropathy is a pathologic change in the kidney that reduces kidney function and leads to kidney failure. Diabetes is the leading cause of end-stage kidney disease (ESKD) and kidney failure in the United States. Risk factors for nephropathy include a 10- to 15-year history of DM, diabetic retinopathy, poor blood glucose control, uncontrolled hypertension, and genetic predisposition. Studies have shown that the onset of diabetic kidney disease may be prevented and the progression to ESKD can be delayed by maintaining optimum blood glucose control, keeping blood pressure within the normal ranges, and using drug therapy to protect the kidneys (American Association of Diabetic Educators [AADE], 2009a). Drugs that protect the kidneys are the angiotensin-converting enzyme (ACE) inhibitors and the angiotensin receptor blockers (ARBs).
Kidney disease causes progressive albumin excretion and a declining glomerular filtration rate (GFR). The earliest manifestation of nephropathy is microalbuminuria (small amounts of albumin in the urine). Annual testing for microalbuminuria is recommended for patients who have had type 1 DM for at least 5 years and in everyone with type 2 DM (ADA, 2010b).
Chronic high blood glucose levels cause hypertension in kidney blood vessels and excess kidney perfusion. The increased pressure damages the kidney in many ways. The blood vessels become leakier, especially in the glomerulus. This leakiness allows filtration of larger particles (including albumin and other proteins), which then form deposits in the kidney tissue and blood vessels. Blood vessels narrow, decreasing kidney oxygenation and leading to kidney cell hypoxia and cell death. These processes worsen over time, with scarring of glomerular blood vessels and loss of urine filtration ability, leading to kidney failure.
Kidney damage is also related to hypertension for patients with DM and cardiovascular disease. Both systolic and diastolic hypertension speed the progression of diabetic nephropathy.
Male Erectile Dysfunction.
Erectile dysfunction (ED) is the inability to achieve or maintain a penile erection sufficient for satisfactory sexual performance. ED occurs at a higher rate (about 50%) and an earlier age among men with DM as compared with the general population. This occurs 10 to 15 years earlier than in the general population and increases with age. It is related to poor blood glucose control, obesity, hypertension, heavy cigarette smoking, and the presence of other chronic vascular complications. Chapter 75 discusses erectile function problems in depth.
Etiology and Genetic Risk
Type 1 Diabetes
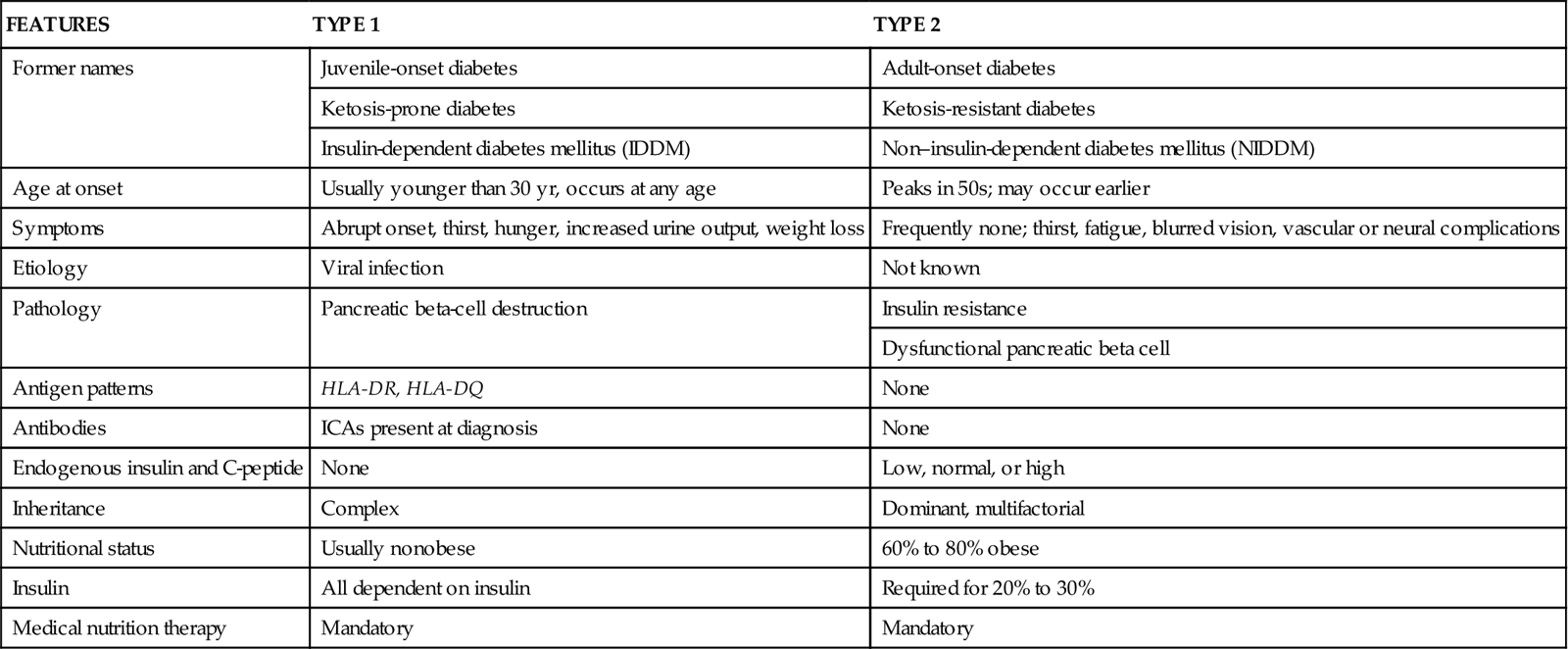
Type 1 diabetes mellitus (DM) is an autoimmune disorder in which beta cells are destroyed in a genetically susceptible person (Table 67-4). The immune system fails to recognize normal body cells as “self” and takes destructive actions against them. In type 1 DM, immune system cells, mediators, and antibodies attack and destroy insulin-secreting cells in the islets. Although the exact cause of why normal cells are attacked by immune system cells is not known, people with certain tissue types are more likely to develop autoimmune diseases, including type 1 DM. Specifically, patients who have the tissue types HLA-DR or HLA-DQ are at an increased risk for type 1 DM. Certain viral infections, such as mumps and coxsackievirus infection, appear to trigger autoimmune destruction of pancreatic beta cells (McCance et al., 2010).
TABLE 67-4
DIFFERENTIATION OF TYPE 1 AND TYPE 2 DIABETES
| FEATURES | TYPE 1 | TYPE 2 |
| Former names | Juvenile-onset diabetes | Adult-onset diabetes |
| Ketosis-prone diabetes | Ketosis-resistant diabetes | |
| Insulin-dependent diabetes mellitus (IDDM) | Non–insulin-dependent diabetes mellitus (NIDDM) | |
| Age at onset | Usually younger than 30 yr, occurs at any age | Peaks in 50s; may occur earlier |
| Symptoms | Abrupt onset, thirst, hunger, increased urine output, weight loss | Frequently none; thirst, fatigue, blurred vision, vascular or neural complications |
| Etiology | Viral infection | Not known |
| Pathology | Pancreatic beta-cell destruction | Insulin resistance |
| Dysfunctional pancreatic beta cell | ||
| Antigen patterns | HLA-DR, HLA-DQ | None |
| Antibodies | ICAs present at diagnosis | None |
| Endogenous insulin and C-peptide | None | Low, normal, or high |
| Inheritance | Complex | Dominant, multifactorial |
| Nutritional status | Usually nonobese | 60% to 80% obese |
| Insulin | All dependent on insulin | Required for 20% to 30% |
| Medical nutrition therapy | Mandatory | Mandatory |
Indicators or markers of immune damage to insulin-producing cells (a key feature of type 1 DM) are the presence of blood antibodies directed against the beta cells themselves or against substances made by beta cells. Most patients with type 1 diabetes have islet cell antibodies (ICAs), insulin autoantibodies (IAAs), autoantibodies to glutamic acid decarboxylase (GAD), or autoantibodies to tyrosine phosphates. Circulating ICA and IAA may be present before manifestations of type 1 DM develop.
Type 2 Diabetes and Metabolic Syndrome
Type 2 DM is a progressive disorder in which the person has a combination of insulin resistance and decreased secretion of insulin by pancreatic beta cells. Insulin resistance (a reduced ability of cells to respond to insulin) develops from obesity and physical inactivity in a genetically susceptible person. Insulin resistance occurs before the onset of type 2 DM and often is accompanied by other cardiovascular risk factors of hyperlipidemia, hypertension, and increased clot formation. Most patients with type 2 DM are obese. With the increased rate of obesity occurring in younger people, the age of onset for type 2 DM is also decreasing. The specific causes of type 2 DM are not known, although insulin resistance and beta-cell failure have many genetic and nongenetic causes. Heredity plays a major role in the development of type 2 DM. Offspring of patients with type 2 DM have a 15% chance for developing the disease and a 30% risk for having impaired glucose tolerance. Specific gene defects have been identified in certain groups with high incidence rates of type 2 DM (Nussbaum et al., 2007).
Metabolic syndrome, also called syndrome X, is the simultaneous presence of metabolic factors known to increase risk for developing type 2 DM and cardiovascular disease. Features of the syndrome include:
Any one of these health problems increases the rate of atherosclerosis and the risk for stroke, coronary heart disease, and early death. Teach patients about the lifestyle changes that can improve health. Reducing weight to within 20% of ideal or body mass index to less than 25 kg/m2 by modifying diet and exercising more will reduce cardiovascular risk. Drug therapy may be required to achieve desired lipid and blood pressure outcomes.
Incidence/Prevalence
More than 57 million American adults have prediabetes, defined as impaired fasting glucose (IFG) or impaired glucose tolerance (IGT). IFG (fasting plasma glucose levels of 100 mg/dL [5.6 mmol/L] to 125 mg/dL [6.9 mmol/L]) and IGT (2-hr oral glucose tolerance values of 140 mg/dL [7.8 mmol/L] to 199 mg/dL [11.0 mmol/L]) are considered risk factors for diabetes and for cardiovascular disease. Over a 3- to 5-year period, people with prediabetes have a fivefold to fifteenfold higher risk for developing type 2 DM than do those with normal blood glucose levels. IFG and IGT are associated with obesity (especially abdominal or central obesity), dyslipidemia with high triglycerides and/or low HDL cholesterol, and hypertension (AADE, 2009b).
The Diabetes Prevention Trial demonstrated that changes in physical activity and eating habits (primarily reduced calories from fat) resulting in weight loss were associated with reduced diabetes risk. Encourage the person with prediabetes to adopt healthy eating habits that limit caloric and fat intake sufficient for weight reduction and to participate in physical activity in order to sustain weight loss.
Diabetes is the seventh leading cause of death in the United States, where it affects 25.8 million people, or 8.3% of the population. An additional 7.0 million people are unaware they have the disease (ADA, 2011).
About 90% of people with diabetes have type 2 DM. It is diagnosed most often among middle-aged and older adults, affecting about 9.6% of patients ages 20 to 59 years and 20.9% of patients ages 60 years or older. The prevalence of diabetes is higher for men than for women (ADA, 2011).
Although type 2 diabetes is a disease of middle-aged and older adults, recent surveys show an increase of the disorder in childhood and adolescence as a result of obesity. Because the prevalence of obesity is rising in North America, diabetes will become even more common. Obesity and a higher-than-normal body mass index (BMI) greatly increase the risk for diabetes.
Health Promotion and Maintenance
Diabetes is a common disorder causing many preventable but devastating complications and is a major public health problem. Control of diabetes and its complications is a major focus for health promotion activities. No interventions are successful in preventing type 1 DM. Health promotion for patients with type 1 DM focuses on controlling hyperglycemia to reduce its long-term complications.
Adopting a low-calorie diet that results in weight loss and increasing physical activity have been shown to improve metabolic and cardiac risk factors. These improvements include reducing hypertension, increasing heart rate variability between resting rate and exercise rate, lowering triglyceride levels, increasing high-density lipoprotein cholesterol (the “good” cholesterol) levels, and reducing low-density lipoprotein cholesterol (the “bad” cholesterol) levels. These changes reduce the incidence of type 2 DM in older adults by 58% (Mozaffarian et al., 2009).
Teach all patients with DM that tight control of blood glucose levels can prevent many complications. Urge all patients with DM to regularly follow up with their health care provider or endocrinologist, to have their eyes and vision tested yearly by an ophthalmologist, and to have urine microalbumin levels assessed yearly. Early detection of changes in the eye or kidney permits adjustments in treatment regimens that can slow or halt progression of retinopathy and nephropathy. Encourage all people to maintain weight within an appropriate range for height and body build and to engage in physical activity at least three times per week.